The best overview of Anthophyta (Angiosperm) Phylogeny
ever published:
Section contents:
1. Flowers
2. Life cycle
3. Pollination
4. Fruits
6. Overview of angiosperm phylogeny
Feature image. Examples of members of the major groups of angiosperms (flowering plants) discussed on this page. Top row, left to right: Amborella trichopoda branch with leaves and flowers (ANA-grade, Amborellales); forked fanwort flower (Cabomba furcata, ANA-grade, Nymphaeales); Austrobaileya scandens flower (ANA-grade, Austrobaileyales); Sarcandra flowers and leaves (Chloranthales). Bottom row, left to right: Japanese spicebush fruits (Lindera triloba, Magnoliids); turquoise puya flower (Puya berteroniana, monocots); hornwort leaves (Ceratophyllum demersum, Ceratophyllales); frangipani flowers (Plumeria rubra, eudicots).
Credits: Amborella trichopoda (Mike Bayly, via Wikimedia Commons, CC BY-SA 3.0); forked fanwort/Cabomba furcata (Bernard DuPont, via flickr, CC BY-SA 2.0); Austrobaileya scandens (Plant Image Library, via flickr, CC BY-SA 2.0); Sarcandra (Aparajita Datta, via Wikimedia Commons, CC BY-SA 4.0); Lindera triloba fruits (Alpsdake, via Wikimedia Commons, CC BY-SA 4.0); Puya berteroana (Wendy Cutler, via Wikimedia Commons, CC BY 2.0); Ceratophyllum demersum (Stefan.Iefnaer, via Wikimedia Commons, CC BY-SA 4.0); Plumeria rubra (SAplants, via Wikimedia Commons, CC BY-SA 4.0).
In 1998, a group of botanical researchers who called themselves the Angiosperm Phylogeny Group (APG) proposed their first classification for angiosperms based on the results of molecular phylogenetic analyses (i.e., analyses of molecular sequence data used to build trees of relationships among living angiosperms; see here). They used a consensus tree of angiosperm relationships to provide a framework for their classification. The APG system is focused on the circumscriptions of families, orders, and higher level clades within the angiosperms. The APG classification system has since undergone three revisions, with the most recent (APG IV) published in 2016. APG IV is followed for angiosperm classification throughout this chapter, unless otherwise noted. The APG IV system does not incorporate completely extinct groups, such as the proposed angiosperm family Archaefructaceae, which includes the fossil genus Archaefructus from the Early Cretaceous of China (see here). Nevertheless, the APG IV classification adequately covers enough angiosperm diversity in the fossil record that its use is not a major impediment for paleobotanists.
Angiosperms include over 295,000 living species of plants. Although division of the angiosperm tree into clades for the purposes of classification is somewhat arbitrary, roughly eight major groups can be recognized under the APG IV scheme. Two of these, the monocots and eudicots, represent the vast majority of living angiosperm species. While the others are relatively small groups, some of them are well represented in the fossil record and important in the early history of angiosperm evolution. An overview of the eight groups is provided below; for a more detailed phylogeny and classification scheme, see the bottom of this page.
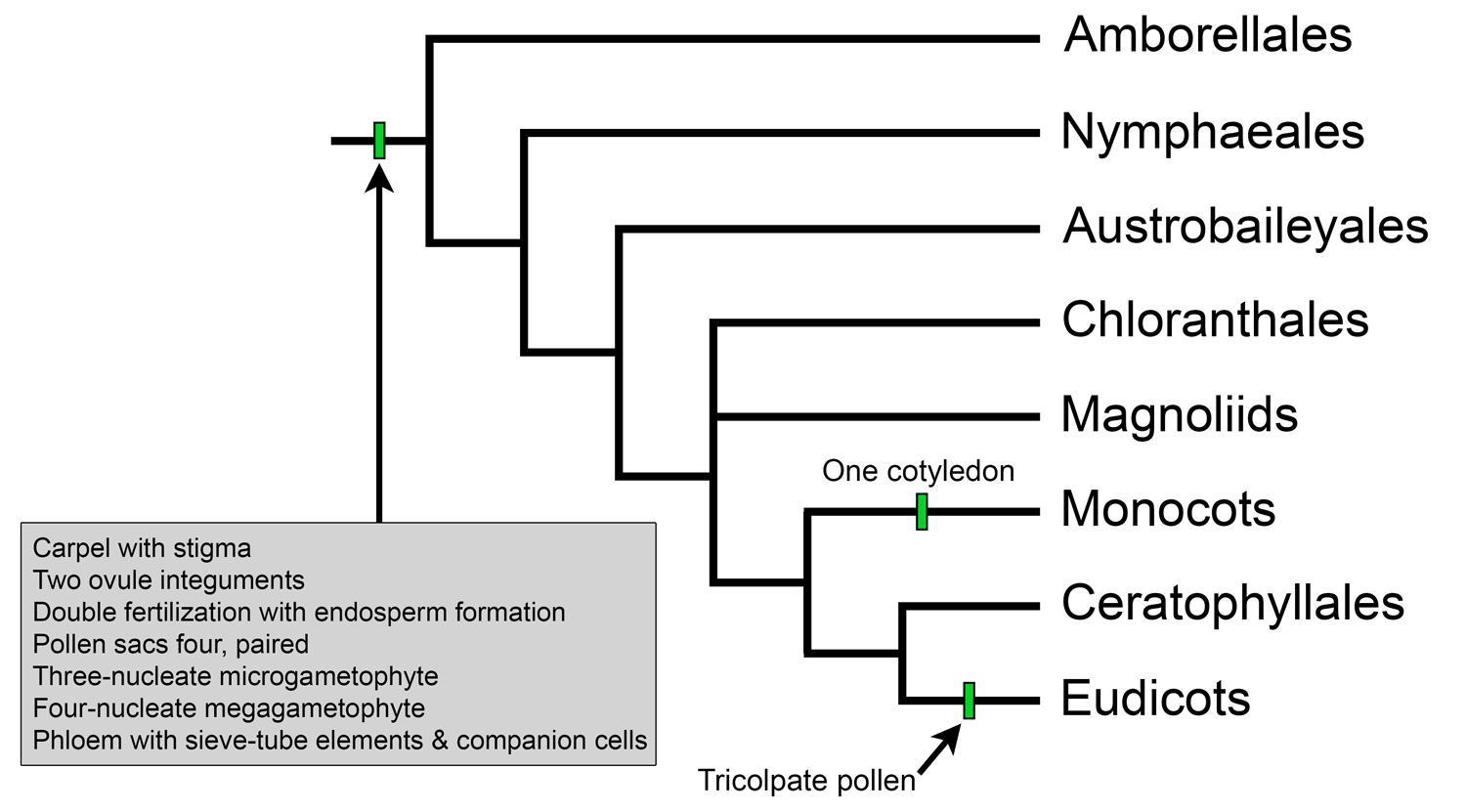
Overview of extant angiosperm relationships based on APG IV. Selected synapomorphies have been mapped on the tree. Credit: Illustration by E.J. Hermsen (DEAL).
ANA grade: Basally diverging groups
The name "ANA grade" is used to collectively refer to the three basally-diverging groups of angiosperms: Amborellales (A), Nymphaeales (N), and Austrobaileyales (A). These groups represent a very small proportion of extant angiosperm diversity, although Nymphaeales and Austrobaileyales are represented in the fossil record beginning in the Cretaceous period. Living ANA-grade angiosperms are significant for helping us to understand some aspects of angiosperm evolution; for example, our understanding of the evolution of the angiosperm megagametophyte (female gametophyte, also called an embryo sac), is based on the study of megagametophytes in members of Nymphaeales and Austrobaileyales (read more here).

Examples of ANA-grade angiosperms. Left. Amborella trichopoda, Amborellaceae, Amborellales. Center. Water lily (Nymphaea), Nymphaeaceae, Nymphaeales. Right. Florida anise tree (Illicium floridanum), Schisandraceae, Austrobaileyales. Credits: Amborella trichopoda (Scott Zona, Wikimedia Commons, CC BY-NC 2.0); Nymphaea (E.J. Hermsen & J.R. Hendricks, DEAL); Illicium floridanum (Scott Zona, Wikimedia Commons, CC BY-NC 2.0). Images modified from originals.
Amborellales
Amborella is a monotypic genus of living plants, which means that it includes only one species: Amborella trichopoda. Amborella trichopoda is also the only species in Amborellaceae and Amborellales. Amborella is native to Grande Terre, New Caledonia, an island located in the Pacific Ocean to the east of Australia. Amborella has been of significant interest to botanists ever since it was widely recognized as sister to all other living angiosperms in the early 21st century on the basis of molecular phylogenetic studies (discussed here and here).
Amborella is a dioecious (Greek, di- + oikos = two houses, meaning plants produce only pollen or ovules) shrub bearing small, unisexual flowers. It retains some apparently ancestral characteristics such as the absence of vessel elements, a type of water-conducting cell characteristic of most angiosperms. Despite this, it does not follow that Amborella generally retains "primitive" or ancestral features, nor that it accurately represents the structure of the earliest angiosperms. (In fact, it has its own unique embryo sac type that is evolved from a simpler ancestral type; see here). Amborella is rather the only living descendent of a lineage that can be traced back to a very ancient divergence in the angiosperm clade, and it is the product of over 100 million years of independent evolution following that divergence. No fossils can be attributed to Amborellales with certainty.

Amborella flowers. Left: Carpellate (female, ovule-producing) flower; carpellate flowers of Amborella have staminodes (sterile stamens). Right: Staminate (male, pollen-producing) flowers. Credits: Amborella trichopoda carpellate flower (Sangtae Kim/Penn State, via flickr, CC BY-NC 2.0); Amborella trichopoda staminate flowers (Scott Zona, via flickr, CC BY-NC 2.0). Images modified from originals.
Nymphaeales
Nymphaeales include three families, Hydatellaceae, Cambombaceae, and Nymphaeaceae (water lilies); the order has 8 living genera and nearly 90 living species. Nymphaeales are rhizomatous, aquatic, freshwater plants that are today found nearly worldwide. Their rich fossil record includes material linked to Cabombaceae and Nymphaeaceae, with the earliest reports being from the Early Cretaceous (for examples, see here and here). Hydatellaceae (1 genus, Hydatella) have inconspicuous, structurally unusual flowers, and no fossils can be unequivocally assigned to the family (see here).

Fossil water lily (Nymphaea) leaf. Leaf of Nymphaea nalinii (Miocene, France). Credit: Nymphaea nalini MNHN 12849 (Jocelyn Falconnet, Muséum National d'Histoire Naturelle, via GBIF, CC BY 4.0).
Austrobaileyales
Austrobaileyales include the families Austrobaileyaceae (Austrobaileya), Schisandraceae (Illicium, Kadsura, and Schisandra), and Trimeniaceae (Trimenia). The order has fewer than 100 species of trees, shrubs, and lianas (woody vines). Austrobaileya and Trimenia are found primarily in Oceania today, whereas Schisandraceae are distributed in eastern North America, the Caribbean, Mexico, Sri Lanka, and eastern to southeastern Asia. Austrobaileyales have a limited fossil record extending to the Early Cretaceous (for example, see here). The most well known member of this group may be the star anise (Illicium verum), which is widely used in Asia as a spice. (It is unrelated to true anise, Pimpinella anisum, a eudicot.)
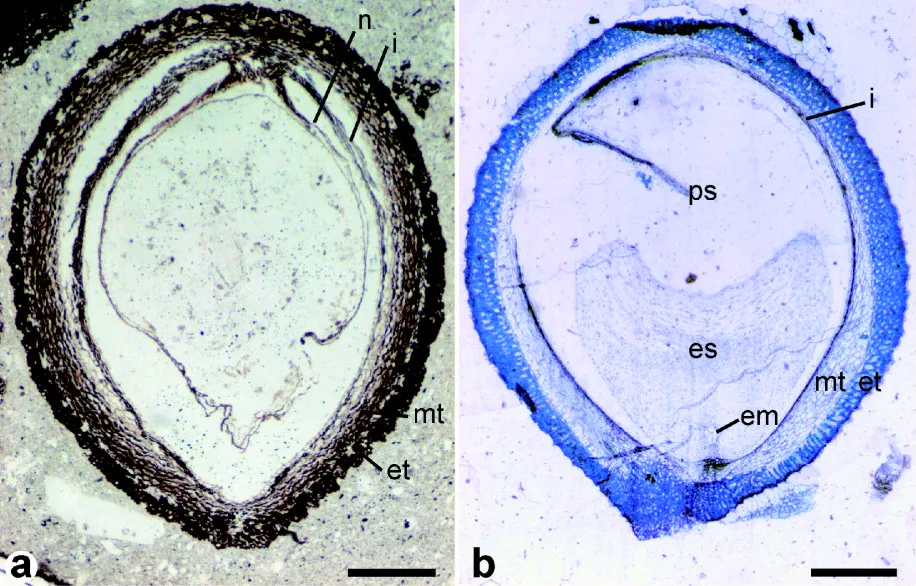
Seeds of fossil and modern Trimeniaceae. Left: Longitudinal section of a fossil Stopesia alveolata seed (Lower Cretaceous, Hikagenosawa Formation, Hokkaido, Japan). Right: Longitudinal section of a modern Trimenia moorei seed. Labels: em = embryo; es, ps = endosperm, perisperm (stored food); et, mt = exotesta & mesotesta (layers of seedcoat); i= inner integument; n = nucellus. Scale bars = 0.5 mm. Credit: From Figure 2 in Yamada et al. (2008) BMC Evolutionary Biology (CC BY 2.0). Image modified from original.
Chloranthales
Chloranthaceae, the only family within the order Chloranthales, includes four living genera and over 70 living species. In some trees depicting relationships amongst angiosperms, this group is resolved as sister to the magnoliids, although this relationship was not recognized in APG IV. Chloranthaceae are distributed in the subtropics to tropics of the Americas, Madagascar, and Asia to southeastern Asia and Pacific islands. Plants in the family can be characterized by, among other attributes, their opposite leaves and small and sessile (stalkless) flowers. Although Chloranthaceae are not terribly diverse today, the family has a significant fossil record beginning in the Early Cretaceous, including macrofossils and pollen (for example, see here).

Examples of plants in Chloranthaceae. Left: Japanese chlorathus (Chloranthus japonicus). Right: Fruits of Sarcandra glabra. Image credits: Chloranthus japonicus (Alpsdake, Wikimedia Commons, CC BY-SA 4.0); Sarcandra glabra (Batholith, Wikimedia Commons, Public Domain). Images modified from originals.
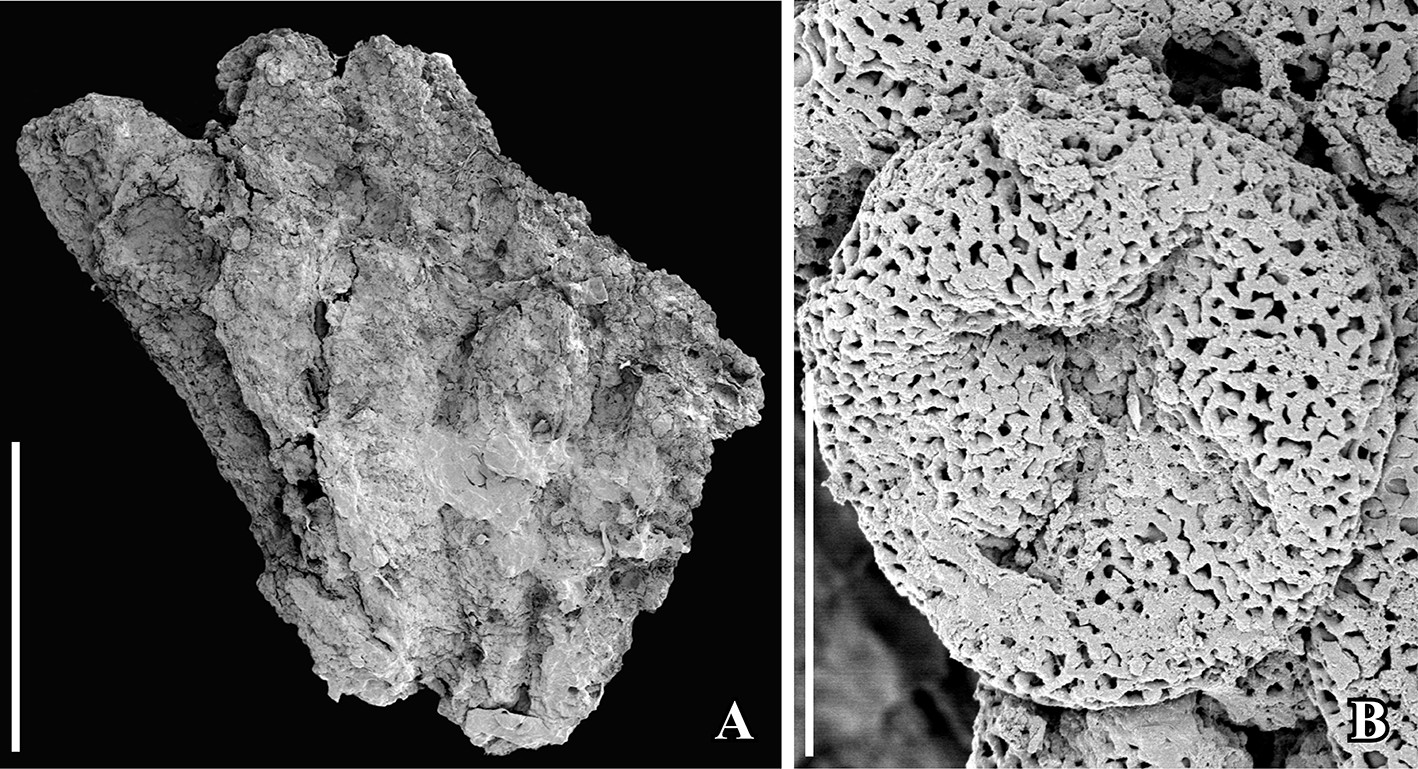
Fossil Chloranthaceae. Scanning electron micrographs (SEM images) of Early Cretaceous (Vale de Água locality, Portugal) fossil Chloranthaceae similar to the modern genus Hedyosmum. Left: Staminate structure (group of stamens). Right: Pollen grain from staminate structure (Asteropollis-type pollen), showing tetrachotomosulcate aperture (i.e., a 4-lobed aperture located on the pole). Credit: From figure 3 in Friis et al. (2019) Int J Plant Sci 180: 232–239 (CC BY-NC 4.0). Image modified from original.
Magnoliids
The magnoliids encompass over 10,000 living species divided amongst four orders: Canellales (2 families, 10 genera), Laurales (7 families, 86 genera), Magnoliales (6 families, 131 genera), and Piperales (3 families, 16 genera). Magnoliids are an eclectic group. From an economic and cultural perspective, some magnoliids are familiar because they are used to make spices due to the presence of ethereal (volatile) oils in their tissues; others produce edible fruits; and some are grown as ornamentals. Selected examples of plants or plant products from this group include:
Laurales: avocado (Persea americana, fruit), bay leaf (Laurus nobilis, herb), cinnamon (several Cinnamomum species, spice), camphor (Cinnamomum camphora, medicinal scent), custard apple and soursop (several Annona species, fruit), pawpaw (Asimina triloba, fruit).
Magnoliales: mace and nutmeg (Myristica fragrans, spice), magnolia (Magnolia spp., ornamental), tulip tree (Liriodendron tulipfera, ornamental, wood), ylang-ylang (Cananga odorata, fragrance).
Piperales: black pepper (Piper nigrum, spice), kava (Piper methysticum, herbal supplement).

Some magnoliids have historically been of interest to botanists due to the presence of characteristics interpreted as primitive in flowering plants. For example, members of Winteraceae (Canellales) have vesselless wood, or wood lacking the vessel elements found in the wood of most angiosperms. In the magnolia family (Magnoliaceae, Magnoliales), flowers have many helically arranged stamens and pistils on an elongated receptacle. In some Magnoliales, stamens are laminar (leaf-like). In Degeneria (Degeneriaceae, Magnoliales) and Drimys (Winteraceae), the carpels are conduplicate (folded) and not completely sealed where their edges meet.

Magnoliids with supposedly primitive flowers. Left: Canelo (Drimys winteri, Canellales), showing central carpels (green structures) surrounded by stamens (yellow structures) and petals (white structures). Right. Section of a carpel of Degeneria (Magnoliales); the edges of the carpel are not fused where they come together (arrow), but are filled with trichomes (hairs). Credits: Canelo/Drimys winteri flowers (Scott Zona, via flickr, CC BY-NC 2.0); Degeneria vitiensis carpel, fig. 91 from Swamy (1949) J Arnold Arbor 30: 10–38 (via Biodiversity Heritage Library, CC BY-NC-SA 3.0). Images modified from originals.
As discussed for Amborella, the idea that some magnoliids have primitive features that provide keys to the early evolution of flowering plants should be treated with caution. Research has indicated, for example, that vesselless wood in Winteraceae may be an adaptation to life in environments that experience freezing temperatures (see here); along with the phylogenetic position of Winteraceae among magnoliid families with vessels, this research suggests that loss of vessels is actually an apomorphy (more recent modification) in Winteraceae rather than a plesiomorphy (characteristic retained from flowering plant ancestors).
The fossil record of magnoliids begins in the Early Cretaceous. A noteworthy extinct member of Magnoliales is Archaeanthus, an early Late Cretaceous plant known from fruits and associated leaves that is similar to the modern magnolia (Magnolia) and tulip tree (Liriodendron) (see here).
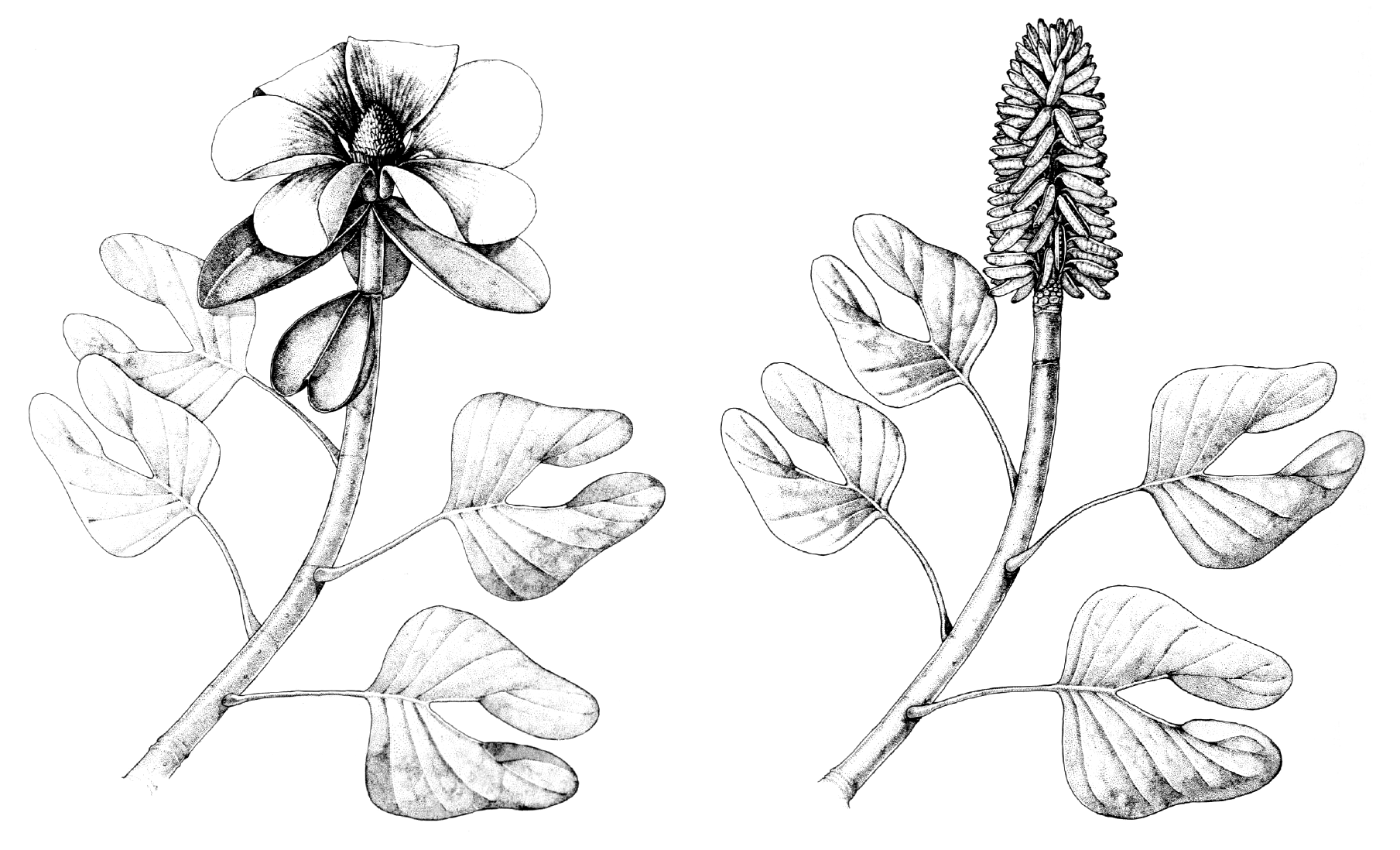
Reconstructions of the Archaeanthus plant. Left: Reconstruction of the Archaeanthus linnenbergeri plant in flower. Right: Reconstruction of the Archaeanthus linnenbergeri plant in fruit. Credits: Reconstructions of the Archaeanthus linnenbergeri plant based on fossils from the Cenomanian (lowermost Upper Cretaceous) Dakota Formation (Kansas, U.S.A.) by Megan Rohn (figs. 69–70 in Dilcher & Crane, 1984, Ann Missouri Bot Gard 71: 351–383, via Biodiversity Heritage Library, CC BY-NC-SA 3.0). Images modified from originals.
Monocots
Moncots are the second largest major clade of angiosperms, after the eudicots, with 11 orders and over 70,000 species. The name monocot is short for "monocotyledon" (Greek, monos = one), a synapomorphy for the group. "Monocotyledon" indicates that the embryo in members of this group has a single cotyledon, or seed leaf. Some additional features typical of monocots are having floral parts in multiples of three, an atactostele in the stem (vascular bundles occur throughout the stem when it is viewed in cross section), and parallel leaf venation. Monocots lack the bifacial vascular cambium that produces wood in many other seed plants, so monocot trees (like palms) must achieve a large circumference through various other methods of growth.

Monocot characteristics. Left: Flower of winding mariposa lily (Calochortus flexuosus) showing floral parts in multiples of three: 3 sepals (not visible), 3 petals, 6 stamens, 3 carpels (indicated by 3 stigma lobes). Right: Cross section of a corn (Zea mays) stem showing an atactostele with scattered vascular bundles (the structures that look like dots or little faces). Credits: Calochortus flexuosus 12 (Stan Shebs, via Wikimedia Commons, CC BY-SA 3.0); Zea mays stem (Jon Houseman and Matthew Ford, via Wikimedia Commons, CC BY-SA 4.0). Images modified from originals.
By far the largest family of monocots, and the largest family of angiosperms overall, is the Orchidaceae (orchids), which includes an estimated 28,000 living species in over 730 genera. The second largest family of monocots is the grass family (Poaceae), with about 12,000 living species in 780 genera. Grasses are the most economically important monocots; cereals such as rice (Oryza), wheat (Triticum), and corn (Zea mays) make up an significant part of the human diet. Examples of other large or notable families of monocots include the arums (Araceae), bromeliads (Bromeliaceae, the group that pineapple is in), gingers (Zingiberaceae), bananas and plantains (Musaceae), lilies (Liliaceae), palms (Arecaceae), and sedges (Cyperaceae).

Examples of monocots. Left: Ram's head lady slipper (Cyprepedium arietinum), orchid family (Orchidaceae). Center: Rattlesnake grass (Briza), grass family (Poaceae). Right: Mexican fan palm (Washingtonia robusta), palm family (Arecaceae). Image credits: Cyprepedium arietinum (James Ellison, Wikimedia Commons, CC BY 2.0); Briza (Alvesgaspar, Wikimedia Commons, CC BY-SA 3.0); Washingtonia robusta (Geographer, Wikimedia Commons, CC BY-SA 3.0). Images modified from originals.
The fossil record of monocots begins in the Early Cretaceous (for example, see here). The quality of the fossil records of monocot orders and familes does not neatly correlate to their present diversity. Orchids are herbaceous (non-woody), are often epiphytes (plants that grow on other plants, from the Greek epi + phyton = upon plant), and have specialized modes of insect pollination that often involve transferring pollen in clumps. Thus, their preservation potential in both the macrofossil and fossil pollen records is low. Orchids have a paltry fossil record with only a few credible occurrences. One interesting way that fossil orchids are preserved is as pollen masses (pollinia) and associated structures stuck to insects trapped in amber (for example, see here).
The richest monocot macrofossil record may belong to the palms (Arecaceae), which arose in the Cretaceous and today include about 180 genera and 2,600 species. Palms have durable tissues in their stems, leaves, and fruits, which increases their potential for preservation. Other monocots that are well represented in the macrofossil record are those that live in wetland or aquatic habitats, like pondweeds (Potomogetonaceae), aquatic arums (Araceae), and cattails (Typhaceae). Fossil monocots are also known from dispersed pollen grains and phytoliths (Greek, phyton + lithos = plant stone), characteristic silica bodies that occur in plant tissues. Pollen and phytoliths are particularly important for the study of grasses, which have a relatively poor macrofossil record.
Slab of fossil palm "wood." Transverse section of a portion of a fossil palm stem (Palmoxylon cheyennense, Cretaceous, Pierre Shale, South Dakota, U.S.A.). Note that palms and other "woody" monocots do not produce true wood. The dots in the palm stem are vascular bundles with associated bundle caps made up of fibers (compare to the cross section of the corn stem shown above). Credit: Model by Emily Hauf (Digital Atlas of Ancient Life, via Sketchfab, CC BY-SA 4.0).

Fossil cattail (Typha) leaves. The fossil leaves of a cattail (Typha latissima, Miocene, France) show the parallel pattern of major veins characteristic of many monocots. Credit: Typha latissima MNHN 12689 (Jocelyn Falconnet, via GBIF, CC BY 4.0).
Ceratophyllales

Hornwort (Ceratophyllum). Left: Hornwort (Ceratophyllum demersum) growing in water. Right: Detail of hornwort (Certatophyllum demersum) showing leaves and staminate inflorescences. Image credits: Left (Radio Tonreg, Wikimedia Commons, CC BY 2.0); right (Christian Fischer, Wikimedia Commons, CC BY-SA 3.0). Images modified from originals.
Ceratophyllum, commonly called hornwort, is the only extant genus in the family Ceratophyllaceae and the order Ceratophyllales; it has four living species. Ceratophyllum is a freshwater aquatic plant that occurs worldwide. It lacks roots and has dissected leaves. Ceratophyllales have a fossil record that may extend to the Early Cretaceous (see here).
Note: Another group of plants commonly called hornworts are placed in the phylum Anthocerotophyta. The hornworts in Anthocerotophyta are bryophytes (nonvascular plants) that are not related to Ceratophyllum. This is yet another example where scientific nomenclature is useful!

Fossil Ceratophyllum. Fossil of Ceratophyllum aquitanicum (Oligocene, France). Credit: Ceratophyllum aquitanicum MNHN 28579 (Jocelyn Falconnet, MNHN-Museum national d'Histoire naturelle, via GBIF, CC BY 4.0). Image modified from original.
Eudicots
The eudicots are by far the largest group of angiosperms. They include over 210,000 species of plants in 44 orders. The term "dicot," short for "dicotyledon," refers to angiosperms that have embryos with two cotyledons, or seed leaves (Greek di- = two). Usage of the term harkens back to an older way of classifying angiosperms, where the angiosperms were split into two major groups: the dicots and the monocots. While the monocots (discussed above) are monophyletic, the dicots as traditionally understood are paraphyletic. In fact, having embryos with two cotyledons is the ancestral (plesiomorphic) condition in angiosperms, as well as in seed plants; cycads, Ginkgo, and gnetophytes all have two cotyledons (the numbers of cotyledons in conifers varies).
The name eudicot, or "true dicot" (Greek eu = good/true), distinguishes the monophyletic eudicot group that includes most dicotyledonous angiosperms from the outdated dicot concept. Eudicots are less commonly called tricolpates because the synapomorphy that defines the group is the production of tricolpate pollen. Such pollen has three elongated or slit-like apertures on its sides. The apertures are thin areas in the pollen wall through which the pollen grain germinates.
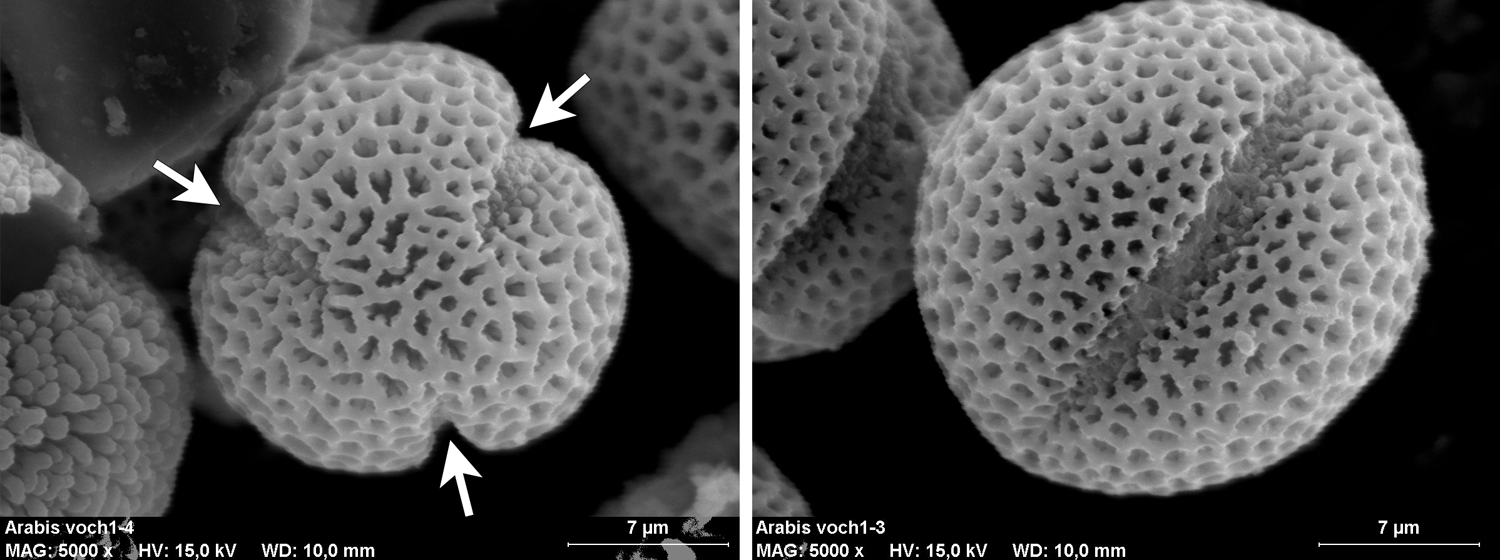
Tricolpate pollen. These scanning electron microscope images come from pollen of rockcress (Arabis), a plant in the mustard family (Brassicaceae). Left: Pollen grain in polar view showing all three apertures (arrows). Right: Pollen grain in equatorial view showing a single aperture. Image credits: Polar view and equatorial view (Maria Majaura, via Wikimedia Commons, CC BY-SA 3.0). Images modified form originals.
Eudicots are often contrasted with monocots based on their vegetative and floral features. Most eudicot characteristics highlighted in such comparisons are ancestral or plesiomorphic within angiosperms (for example, two cotyledons, eustele). One exception is the presence of tricolpate pollen discussed above; monosulcate pollen, the monocot condition, is ancestral (plesiomorphic) within angiosperms. Additionally, floral parts of eudicots are often in multiples of four or five. Flowers with five parts per whorl are a synapomorphy for the Pentapetalae, a large clade that includes most eudicots (= Superrosids + Superasterids; see the diagram at the bottom of this page). Floral parts in multiples of three, typical of monocots, might be the ancestral angiosperm condition (see here).
Comparison table of monocot vs. eudicot characteristics. Note that these characteristics are generalizations, and individual species may deviate in one or more features. Table modified & expanded after table 19-1 in Evert & Eichhorn (2013), Raven Biology of Plants.
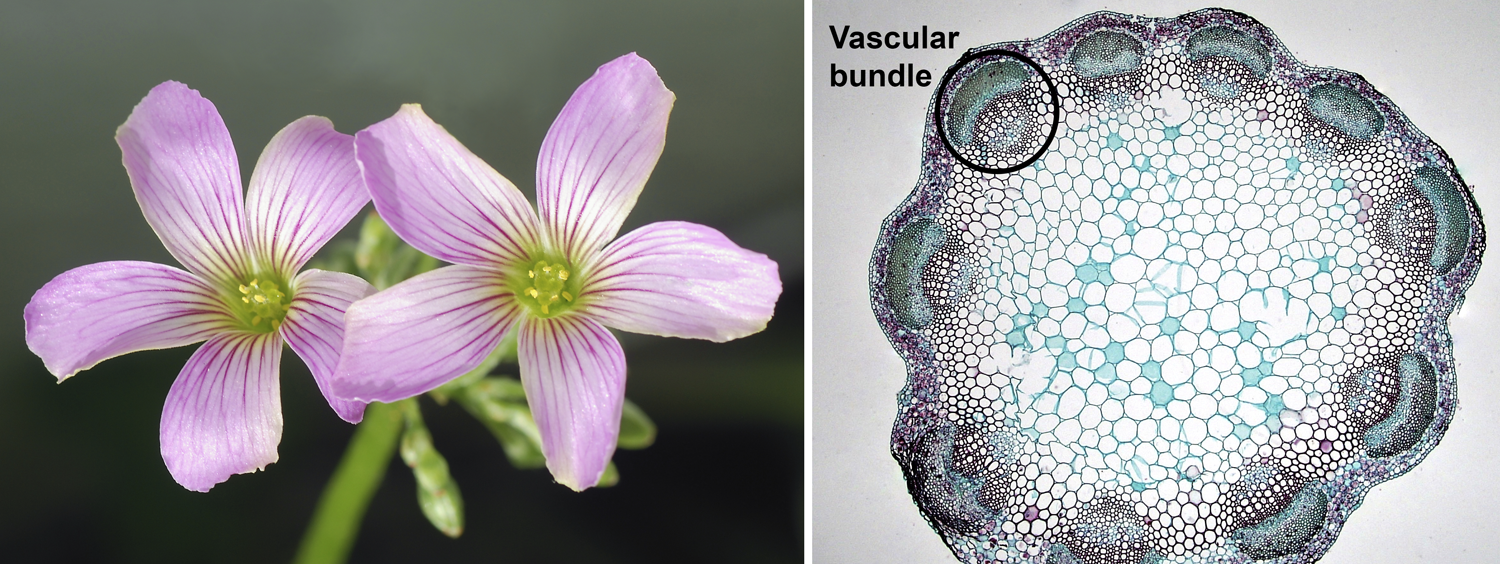
Eudicot characteristics. Left: Flowers of pink woodsorrel (Oxalis debilis), showing 5-parted floral whorls; flowers have 5 sepals (not visible), 5 petals, 10 stamens, and 5 carpels (indicated by 5 stigmas). Right: Cross-section of a clover (Trifolium) stem showing a eustele (vascular bundles in a ring; one bundle is circled). Credits: Oxalis debilis (Alvesgaspar, via Wikimedia Commons, CC BY-SA 3.0); young Trifolium stem cross-section (Berkshire Community College Bioscience Image Library, via flickr, CC 0 1.0/Public Domain).

Eudicot fossils. Left: Five-parted calyx of a fossil member of the mallow family (Florissantia quichenensis, Malvaceae); size = 2.9 cm in diameter. Right: Leaf of a fossil member of the witch hazel family (Langeria magnifica, Hamamelidaceae) showing pinnate venation pattern with a midrib and large lateral veins (pinnate = feather-like); minor veins form a net-like reticulum. Both fossils are from the Ypresian/lower Eocene Republic flora, Klondike Mountain Formation, Washington, U.S.A. Credits: Florissantia quichenensis (Kevmin, via Wikimedia Commons, CC BY-SA 3.0); Langeria magnifica (Kevmin, via Wikimedia Commons, CC BY-SA 4.0). Images modified from originals.
Eudicots include five basally-diverging orders (Ranunculales, Proteales, Trochodendrales, Buxales, and Gunnerales) and two major clades: the Superrosids (19 orders) and Superasterids (20 orders). The diversity of eudicots is so great that the group is difficult to summarize. Three large families account for over a quarter (>25%) of living eudicot species. These include:
Asteraceae: Asteraceae (aster, daisy, or sunflower family) include over 1,600 living genera and an estimated 24,700 living species. Examples are daisies (plants in multiple genera), chrysanthemums (Chrysanthemum), goldenrods (Solidago), sunflowers (Helianthus), and zinnias (Zinnia).
Fabaceae: The legume family, Fabaceae, includes about 750 living genera and 19,500 living species. Examples are beans (multiple genera), lupines (Lupinus), peanut (Arachis hypogea), peas (multiple genera), soybean (Glycine max), and tamarind (Tamarindus indica).
Rubiaceae: Rubiaceae (bedstraw, coffee, or madder family) have about 590 living genera and over 13,600 living species. The most familiar member of this family is, obviously, coffee (Coffea). Gardenia (Gardenia) is grown as an ornamental.

Examples of eudicots. Left: A sunflower (Helianthus), aster family (Asteraceae). Center: Jade vine (Strongylodon macrobotrys), legume family (Fabaceae). Right: Bluets (Houstonia), coffee family (Rubiaceae). Image credits: Sunflower (Helianthus) and bluets (Houstonia) by E.J. Hermsen (DEAL); jade vine (Strongylodon) by J.R. Hendricks (DEAL).
Examples of other types of eudicots include birches (Betula), buttercups (Ranunculus), cacti (Cactaceae), grapes (Vitis), gourds (Cucurbitaceae), mustards (Brassicaceae), nightshades (Solanaceae), oaks (Quercus), roses (Rosa), and willows (Salix). Cotton (Gossypium), apples (Malus), citrus fruits (Citrus), olive (Olea europaea), tea (Camellia sinensis), tobacco (Nicotiana tabacum), and cannabis (Cannabis) are eudicots.
As in other major angiosperm groups, the earliest evidence for eudicots appears in the Early Cretaceous; it consists of dispersed, tricolpate pollen grains (reviewed here). Early Cretaceous macrofossil remains of eudicots are also known (for example, see here and here). The eudicots diversified rapidly, and plants related to modern families and genera are recognizable in the Late Cretaceous.
As in the monocots, the present diversity of eudicot groups does not necessarily correlate to their representation in the fossil record. For example, the family Platanaceae (plane-tree family) includes one living genus (Platanus, sycamores and plane trees) with about 8 living species. However, the family has a good fossil record beginning in the Early Cretaceous (partially reviewed here) that includes extinct genera like Paleogene taxon Macginitea. The quality of the fossil record may be due to the structural features of plane-trees, like robust leaves and dry fruits. In contrast, Asteraceae (the aster family), the largest extant eudicot family by number of species, are known from pollen but are poorly represented in the macrofossil record (reviewed here and here).

Fossil sycamores (Plantanaceae). Left: Macginitea wyomingensis leaf (Bonanza locality, Eocene, Parachute Creek Member of the Green River Formation, Utah, U.S.A.). Right: Macginicarpa manchesteri fruit; fruit type = multiple of achenes (near Red Deer River, Paleocene, Paskapoo Formation, Alberta, Canada). Credits: Macginitea wyomingensis, YPM PB 168143 (Division of Paleobotany, Yale Peabody Museum, via GBIF, CC0 1.0/Public Domain); Fossil Platanus fruit (=Macginicarpon manchesteri; Georgialh, via Wikimedia Commons, CC BY-SA 4.0). Images modified from originals.
Overview of angiosperm phylogeny
The image below provides a detailed overview of angiosperm phylogeny and classification down to the ordinal level. Families included in each order are listed on the right.
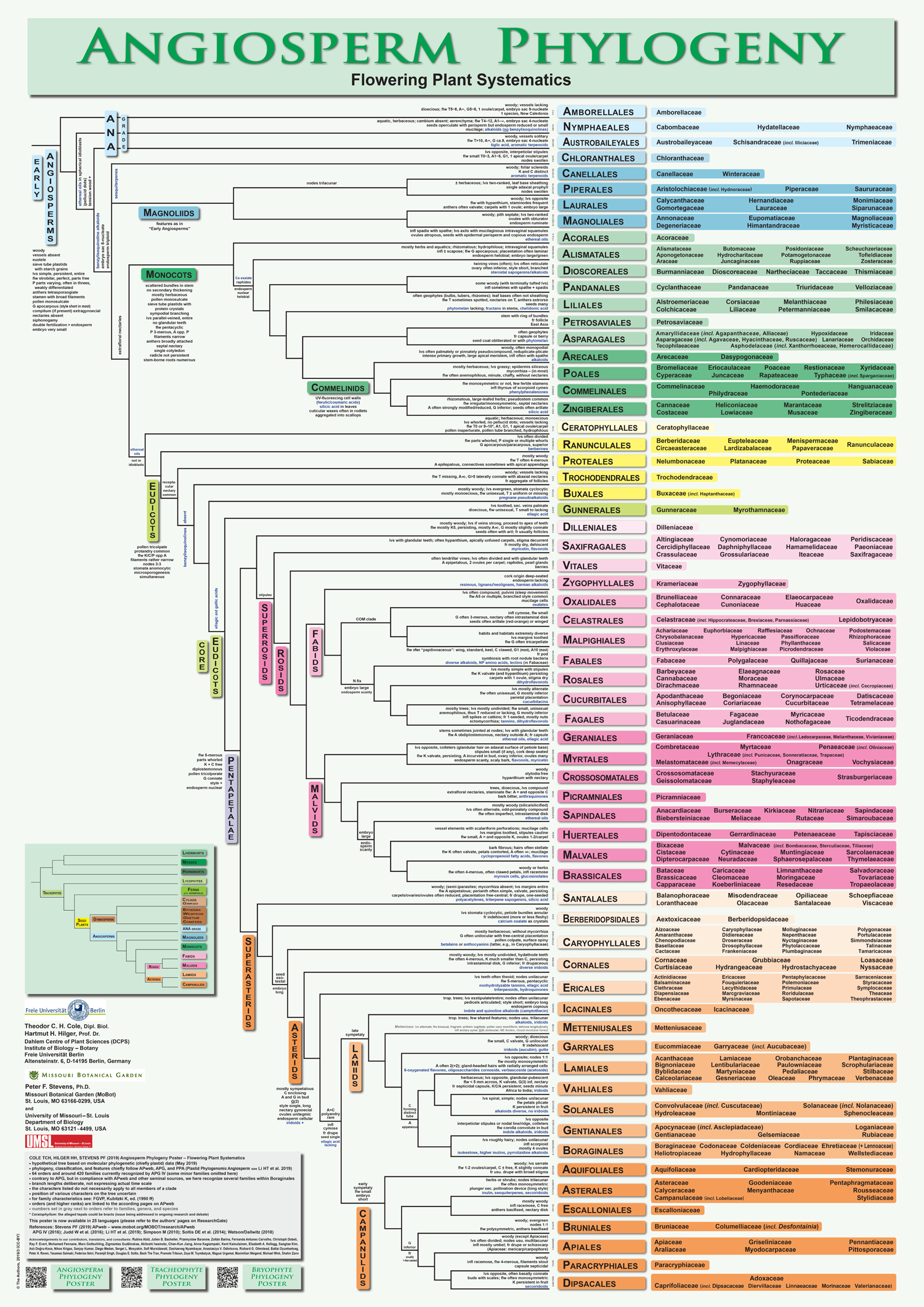
Angiosperm phylogeny and classification. The diagram above provides an overview of extant angiosperm phylogeny and classification based on APG IV and other sources. Credit: Cole et al. (2019) PeerJ Preprints 7: e2320v6 (CC BY 4.0). This image was modified (resized, file format changed). For a full-sized PDF, consult the original.
Selected references
Note: Free full text is made available by the publisher for items marked with a green star.
Academic articles & book chapters
* Angiosperm Phylogeny Group. 1998. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden 85: 531–553. Read/download free at Biodiversity Heritage Library: https://www.biodiversitylibrary.org/part/2234#/summary. Read free on JSTOR (account sign-up or institutional access required): https://doi.org/10.2307/2992015.
* Angiosperm Phylogeny Group. 2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society 141: 399–436. https://doi.org/10.1046/j.1095-8339.2003.t01-1-00158.x
* Angiosperm Phylogeny Group. 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105–121. https://doi.org/10.1111/j.1095-8339.2009.00996.x
* Angiosperm Phylogeny Group, M.W. Chase, M.J.M. Christenhusz, M.F. Fay, J.W. Byng, W.S. Judd, D.E. Soltis, D.J. Mabberley, A.N. Sennikov, P.S. Soltis, and P.F. Stevens. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. https://doi.org/10.1111/boj.12385
* Christenhusz, M.J.M., and J.W. Byng. 2016. The number of known plants species in the world and its annual increase. Phytotaxa 261: 201–217. http://dx.doi.org/10.11646/phytotaxa.261.3.1
Coiffard, C., N. Kardjilov, I. Manke, and M.E.C. Bernardes-de-Oliveira. 2019. Fossil evidence of core monocots in the Early Cretaceous. Nature Plants 5: 691–696. https://doi.org/10.1038/s41477-019-0468-y
Coiffard, C., B.A.R. Mohr, and M.E.C. Bernardes-de-Oliveira. Jaguariba wiersemana gen. nov. et sp. nov., an Early Cretaceous member of crown group Nymphaeales (Nymphaeaceae) from northern Gondwana. Taxon 62: 141–151. https://doi.org/10.1002/tax.621012
* Dilcher, D.L., and P.R. Crane. 1984. Archaeanthus: An early angiosperm from the Cenomanian of the Western Interior of North America. Annals of the Missouri Botanical Garden 71: 351–383. Read/download for free from Biodiversity Heritage Library: https://www.biodiversitylibrary.org/part/3952#/summary. Read free on JSTOR (account sign-up or institutional access required): https://doi.org/10.2307/2399030.
Doyle, J.A. 2013. Phylogenetic analyses and morphological innovations in land plants. In: B.A. Ambrose and M. Purugganan, eds. The evolution of plant form. Annual Plant Reviews 45: 1–50. Blackwell Publishing Ltd. https://doi.org/10.1002/9781118305881
* Feild, T.S., T. Brodribb, and N.M. Holbrook. 2007. Hardly a relict: Freezing and the evolution of vesselless wood in Winteraceae. Evolution 56: 464–478. https://doi.org/10.1111/j.0014-3820.2002.tb01359.x
* Friis, E.M., P.R. Crane, and K.R. Pedersen. 2019. Hedyosmum-like fossils in the Early Cretaceous diversification of angiosperms. International Journal of Plant Sciences 180: 232–239. https://doi.org/10.1086/701819
* Friis, E.M., M.M. Mendes, and K.R. Pedersen. 2017. Paisia, an Early Cretaceous eudicot angiosperm flower with pantoporate pollen from Portugal. Grana 57: 1–15. https://doi.org/10.1080/00173134.2017.1310292
Kvaček, Z., S.R. Manchester, and S.-x. Guo. 2001. Trifoliate leaves of Platanus bella (Heer) comb. n. from the Paleocene of North America, Greenland, and Asia and their relationships among extinct and extant Platanaceae. International Journal of Plant Sciences 162: 441–458. https://doi.org/10.1086/319574
Manchester, S.R., F. Grímsson, and R. Zetter. 2015. Assessing the fossil record of Asterids in the context of our current phylogenetic framework. Annals of the Missouri Botanical Garden 100: 329–363. https://doi.org/10.3417/2014033. Read free on PubMed Central, PMC ID PMC6485501
Martínez-Millán, M. 2010. Fossil record and age of the Asteridae. The Botanical Review 76: 83–135. https://doi.org/10.1007/s12229-010-9040-1
Mohr, B.A.R., M.E.C. Bernardes-de-Oliveira, and D.W. Taylor. 2008. Pluricarpellatia, a nymphaealean angiosperm from the Lower Cretaceous of northern Gondwana (Crato Formation, Brazil). Taxon 57: 1147–1158. https://doi.org/10.1002/tax.574009
Pigg, K.B., and R.A. Stockey. 1991. Platanaceous plants from the Paleocene of Alberta, Canada. Review of Palaeobotany and Palynology 70: 125–146. https://doi.org/10.1016/0034-6667(91)90082-E
* Poinar, G., Jr., Rasmussen, F.N. 2017. Orchids from the past, with a new species in Baltic amber. Botanical Journal of the Linnean Society 183: 327–333. https://doi.org/10.1093/botlinnean/bow018
Punt, W., P.P. Hoen, S. Blackmore, S. Nilsson, and A. Le Thomas. 2007. Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology 143: 1–81. https://doi.org/10.1016/j.revpalbo.2006.06.008
* Rudall, P.J., D.D. Sokoloff, M.V. Remizowa, J.G. Conran, J.I. Davis, T.D. Macfarlane, and D.W. Stevenson. 2007. Morphology of Hydatellaceae, an anomolous aquatic family recently recognized as an early-divergent angiosperm lineage. American Journal of Botany 94: 1073–1092. https://doi.org/10.3732/ajb.94.7.1073
* Sauquet, H., M. von Balthazar, S. Magallón, J.A. Doyle, P.K. Endress, E.J. Bailes, E. Barroso de Morais, K. Bull-Hereñu, L. Carrive, M. Chartier, G. Chomicki, M. Coiro, R. Cornette, J.H. L. El Ottra, C. Epicoco, C.S.P. Foster, F. Jabbour, A. Haevermans, T. Haevermans, R. Hernández, S.A. Little, S. Löfstrand, J.A. Luna, J. Massoni, S. Nadot, S. Pamperl, C. Prieu, E. Reyes, P. dos Santos, K.M. Schoonderwoerd, S. Sontag, A. Soulebeau, Y. Staedler, G.F. Tschan, A.W.-S. Leung, J. Schönenberger. 2017. The ancestral flower of angiosperms and its early diversification. Nature Communications 8: 16047. https://doi.org/10.1038/ncomms16047
* Soltis, P.S., R.A. Folk, and D.E. Soltis. 2019. Darwin review: angiosperm phylogeny and evolutionary radiations. Proceedings of the Royal Society B, Biological Sciences 286: 20190099. https://doi.org/10.1098/rspb.2019.0099
Sun, G., Q. Ji, D.L. Dilcher, S. Zheng, K.C. Nixon, and X. Wang. 2002. Archaefructaceae, a new basal angiosperm family. Science 296: 899–904. https://doi.org/10.1126/science.1069439
* Swamy, B.G.L. 1949. Further contributions to the morphology of the Degeneriaceae. Journal of the Arnold Arboretum 30: 10–38. Read/download free from Biodiversity Heritage Library: https://www.biodiversitylibrary.org/part/185600#/summary
* Wang, W., D.L. Dilcher, H.-S. Wang, and Z.-D. Chen. 2014. Accelerated evolution of early angiosperms: Evidence from ranunculalean phylogeny by integrating living and fossil data. Journal of Systematics and Evolution 54: 336–341. https://doi.org/10.1111/jse.12090
Tschan, G.F., T. Denk, and M. von Balthazar. 2008. Credneria and Platanus (Plantanaceae) from the Late Cretaceous (Santonian) of Quedlinburg, Germany. Review of Palaeobotany and Palynology 152: 211–236. https://doi.org/10.1016/j.revpalbo.2008.05.004
Wang, H., and D.L. Dilcher. 2018. A new species of Donlesia (Ceratophyllaceae) from the Early Cretaceous of Kansas, U.S.A. Review of Palaeobotany and Palynology 252: 20–28. https://doi.org/10.1016/j.revpalbo.2018.02.002
* Yamada, T., and M. Kato. 2008. Stopesia alveolata, a fossil seed of Trimeniaceae from the Lower Cretaceous (Albian) of Hokkaido, northern Japan. Acta Phytotaxonomica et Geobotanica 59: 228–230. https://doi.org/10.18942/apg.KJ00005124838
* Yamada, T., H. Nishida, M. Umebayashi, K. Uemura, and M. Kato. 2008. Oldest record of Trimeniaceae from the Early Cretaceous of northern Japan. BMC Evolutionary Biology 8: article 135. https://doi.org/10.1186/1471-2148-8-135
Angiosperm phylogeny poster
* Cole, T.C.H., H.H. Hilger, and P. Stevens. 2019. Angiosperm phylogeny poster (APP) – Flowering plant systematics, 2019. PeerJ Preprints 7: e2320v6. https://doi.org/10.7287/peerj.preprints.2320v6
Books & textbooks
Evert, R.F., and S.E. Eichhorn. 2013. Raven Biology of Plants, 8th ed. W.H. Freeman and Co., New York, New York.
Friis, E.M., P.R. Crane, and K.R. Pedersen. 2011. Early Flowers and Angiosperm Evolution. Cambridge University Press, Cambridge, U.K.
Foster, A.S., and E.M. Gifford. 1974. Comparative Morphology of Vascular Plants, 2nd ed. W.H. Freeman and Co., San Francisco.
Simpson, M.G. 2010. Plant Systematics, 2nd ed. Academic Press, Burlington, Massachusetts.
Websites
* B.R.S. 1995. Monocots versus dicots, the two classes of flowering plants. 1995. University of California Museum of Paleontology. https://ucmp.berkeley.edu/glossary/gloss8/monocotdicot.html
* Soltis, P., D. Soltis, and C. Edwards. 2005. Angiosperms. Flowering Plants. ver. 03, 3 June 2005. In: The Tree of Life Web Project (http://tolweb.org). http://tolweb.org/Angiosperms/20646/2005.06.03
* Soltis, P., D. Soltis, and C. Edwards. 2005. Magnoliids. ver. 23 June 2005. In: The Tree of Life Web Project. (http://tolweb.org). http://tolweb.org/magnoliids/20670
* Stevens, P.F. 2001 onwards. Angiosperms Phylogeny Website. http://www.mobot.org/MOBOT/research/APweb/welcome.html
Content usage
Usage of text and images created for DEAL: Text on this page was written by Elizabeth J. Hermsen. Original written content created by E.J. Hermsen for the Digital Encyclopedia of Ancient Life that appears on this page is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Original images and diagrams created by E.J. Hermsen are also licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Content sourced from other websites: Attribution, source webpage, and licensing information or terms of use are indicated for images sourced from other websites in the figure caption below the relevant image. See original sources for further details. Attribution and source webpage are indicated for embedded videos. See original sources for terms of use. Reproduction of an image or video on this page does not imply endorsement by the author, creator, source website, publisher, and/or copyright holder.
Adapted images. Images that have been adapted or remixed for DEAL (e.g., labelled images, multipanel figures) are governed by the terms of the original image license(s) covering attribution, general reuse, and commercial reuse. DEAL places no further restrictions above or beyond those of the original creator(s) and/or copyright holder(s) on adapted images, although we ask that you credit DEAL if reusing an adapted image from the DEAL website. Please note that some DEAL figures may only be reused with permission of the creator(s) or copyright holder(s) of the original images. Consult the individual image credits for further details.
Page first released 10 November 2019; last updated 1 April 2020.
https://www.nature.com/articles/ncomms16047
https://www.annualreviews.org/doi/pdf/10.1146/annurev.ecolsys.110308.120203
https://www.digitalatlasofancientlife.org/learn/embryophytes/tracheophytes/
https://nph.onlinelibrary.wiley.com/doi/pdf/10.1111/nph.13264
INTRODUÇÃO À ANATOMIA DAS
PLANTAS VASCULARES
Feature image. Plant anatomy sections. Left: Cross section of a polyarch root of Aeglopsis chevalieri, a plant in the citrus family (Rutaceae) of angiosperms. Center: Cross section of a woody stem of kenaf (Hibisicus cannabinus, an angiosperm). Right: Epidermis of a leaf of redgal (Morinda royoc, an angiosperm). Credits: Aeglopsis chevalieri Swingle (SV422), Hibiscus cannabinus L. (C8), and Morinda roioc (F24) (University of Miami Swingle Plant Anatomy Reference Collection, not under copyright in the United States). Images modified from originals.
What are vascular plants (tracheophytes)?
Most land plants are tracheophytes, or vascular plants. Their defining feature is the presence of vascular tissue, or specialized tissue that conducts food (phloem) and water (xylem). When considered in a phylogenetic context or in the fossil record, tracheophytes can be more specifically defined by the origin of a type of water-conducting cell known as a tracheid. The first evidence for tracheids shows up in the fossil record in the Silurian period. Tracheophytes later diversified structurally, with many key innovations appearing by the end of the Devonian period, such as leaves and wood. The dominant life stage in most vascular plants is the sporophyte, or the diploid, spore-producing plant.
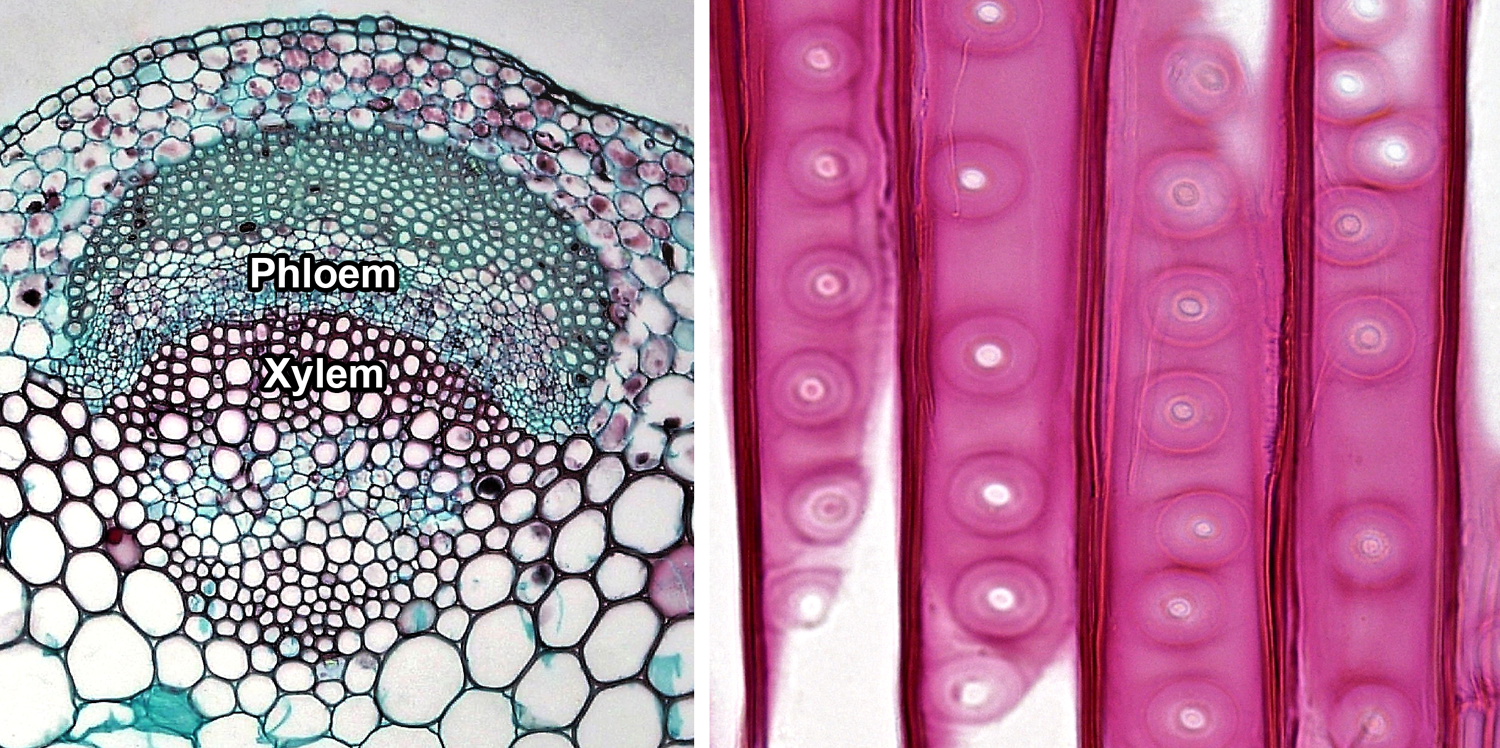
Vascular tissue. Left: Cross section of a vascular bundle in the stem of clover (Trifolium, an angiosperm) showing food-conducting phloem and water-conducting xylem. Right: Detail of portion of the tracheids in pine (Pinus, a conifer) wood, radial section (longitudinal section on the radius of the axis); note the characteristic circular-bordered pits typical of conifer tracheids. Credits: Cortex and pith in Trifolium stem and Circular bordered pits in Pinus wood (Fayette A. Reynolds M.S./Berkshire Community College Bioscience Library, via flickr, CC0 1.0/Public Domain Dedication).
Living tracheophytes belong to one of two major sister groups: 1) The lycophytes (clubmosses and relatives); and 2) The euphyllophytes (ferns, horsetails, and seed plants). The lycophytes, ferns, and horsetails are free-sporing (seedless) vascular plants sometimes collectively called pteridophytes. These plants share certain plesiomorophic or ancestral structural and developmental characteristics. Thus, it is sometimes useful when discussing the comparative sporophyte structure of vascular plants to contrast the paraphyletic pteridophytes with the monophyletic spermatophytes, or seed plants. Furthermore, while angiosperms (flowering plants) are a group of seed plants, they have many of their own unique structural characteristics that set them apart from gymnosperms (the group made up of non-angiospermous seed plants).
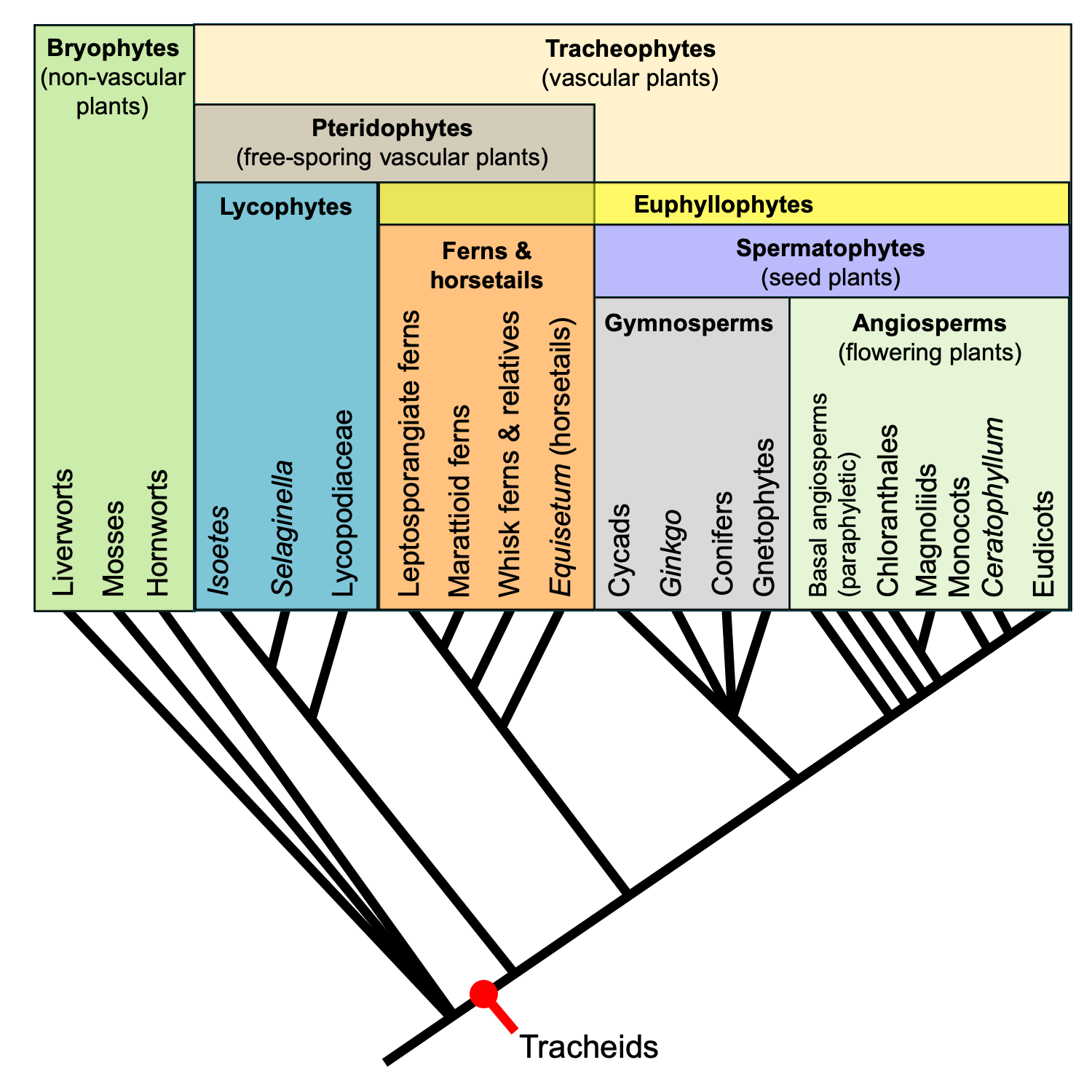
Relationships among major extant land plant groups. This phylogenetic tree shows the relative relationships among extant land plants, with emphasis on vascular plant groups. Note that the relationships among extant gymnosperm groups is shown as unresolved. Recent molecular phylogenetic analyses indicate that extant gymnosperms are monophyletic; this contradicts the traditional view that extant gymnosperms are a paraphyletic group. Regardless, gymnosperms are paraphyletic when extinct groups are included. Credit: E.J. Hermsen (DEAL).
Overview of sporophyte structure
The study of plant structure includes morphology, the study of form, and anatomy, the study of internal structure (cells, tissues, etc.). Paleobotany uses both of these disciplines in order to analyze fossil plants.
Organs & organ systems of the plant body
The vascular plant sporophyte (hereafter, simply called the plant) includes three basic categories of non-reproductive, or vegetative, organs. These are the roots, stems, and leaves. Roots are organs that typically occur belowground and anchor the plant in the soil. They also function in absorbing water and sometimes in storage. Stems are often (although not always) aboveground structures. Stems bear the leaves, and also serve as conduits between roots and leaves. Finally, leaves are lateral appendages on the stem. Their main function is photosynthesis. A stem and its leaves together are known as a shoot.
Plant organs can be grouped into two organ systems. All the shoots (stems and leaves) of a plant are together known as the shoot system, whereas all the roots together comprise the root system. Sporangia (spore-producing structures) are borne on modified leaves or shoots; thus, sporangium-bearing organs can also be considered part of the shoot system.
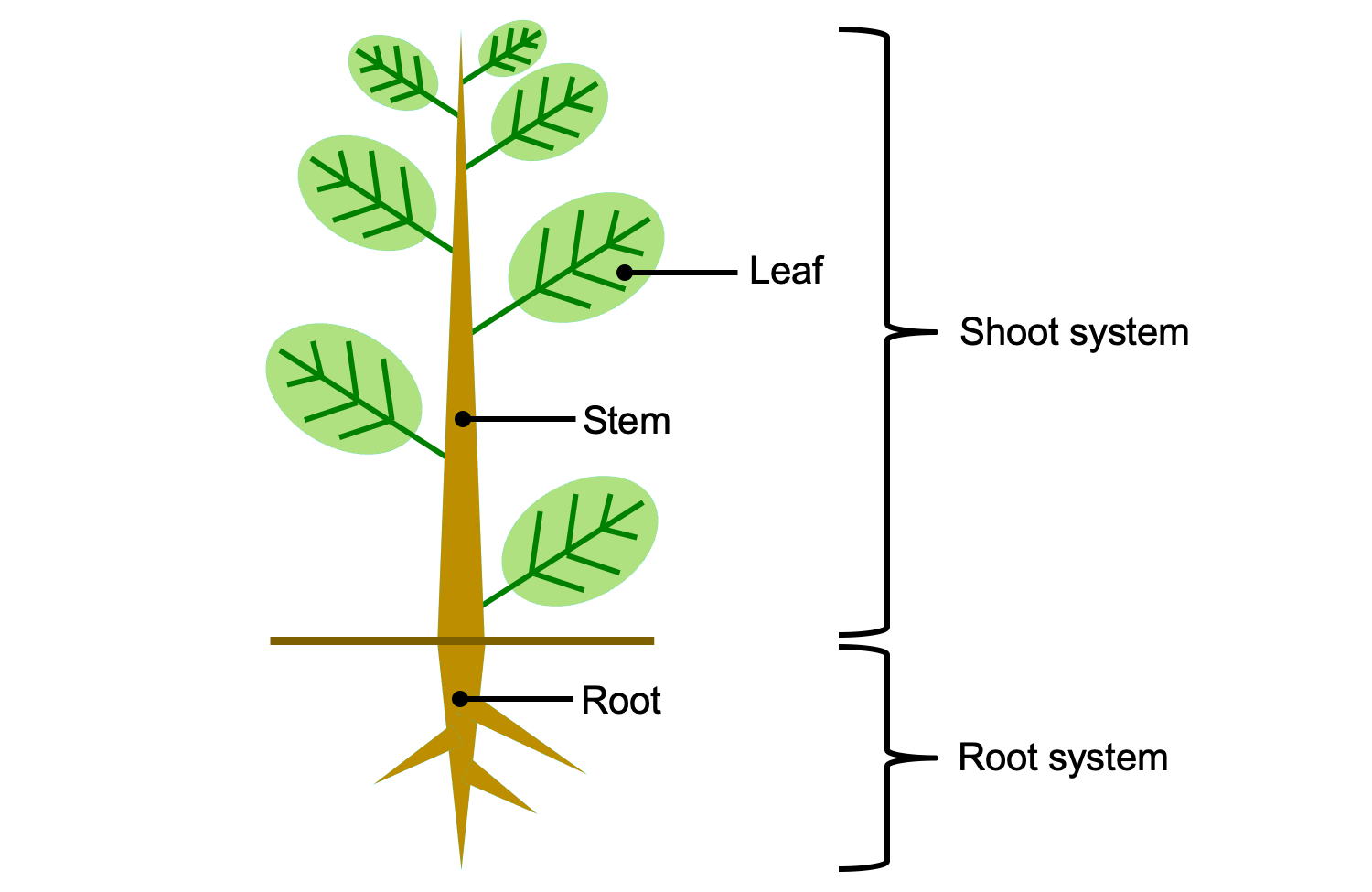
Vegetative organs and organ systems of the vascular plant sporophyte. The shoot system includes al of the stems, leaves, and reproductive structures (sporangia, cones, flowers, etc.). The root system includes all of the roots. Credit: E.J. Hermsen (DEAL).
Growth
Growth is the process by which a plant increases in size (length, height, or girth) through cell division (adding new cells) and cell expansion. Active cell division is typically concentrated in regions known as meristems. As cells are distanced from the meristems by new growth, they begin to differentiate. Differentiation is the process by which a cell takes on a specific identity and function in the plant body. Once a cell is fully differentiated, or mature, it often does not—and in many cases cannot—divide further.
Vascular plant sporophytes have two major categories of meristems that contribute to growth: apical and lateral. However, other types of meristems may contribute to growth of stems and expansion of leaves (e.g., intercalary meristems, marginal meristems).
APICAL MERISTEMS
Apical meristems are meristems at the tips of roots and stems that add length to these organs; growth from apical meristems is known as primary growth. Primary growth also produces the leaves. The primary meristems—protoderm, ground meristem, and procambium—include partially differentiated cells. The primary meristems can be found in regions still undergoing growth between the apical meristem proper and the fully mature tissues of the stem, leaf, and root. They correspond to the three tissue systems of vascular plants (discussed below).
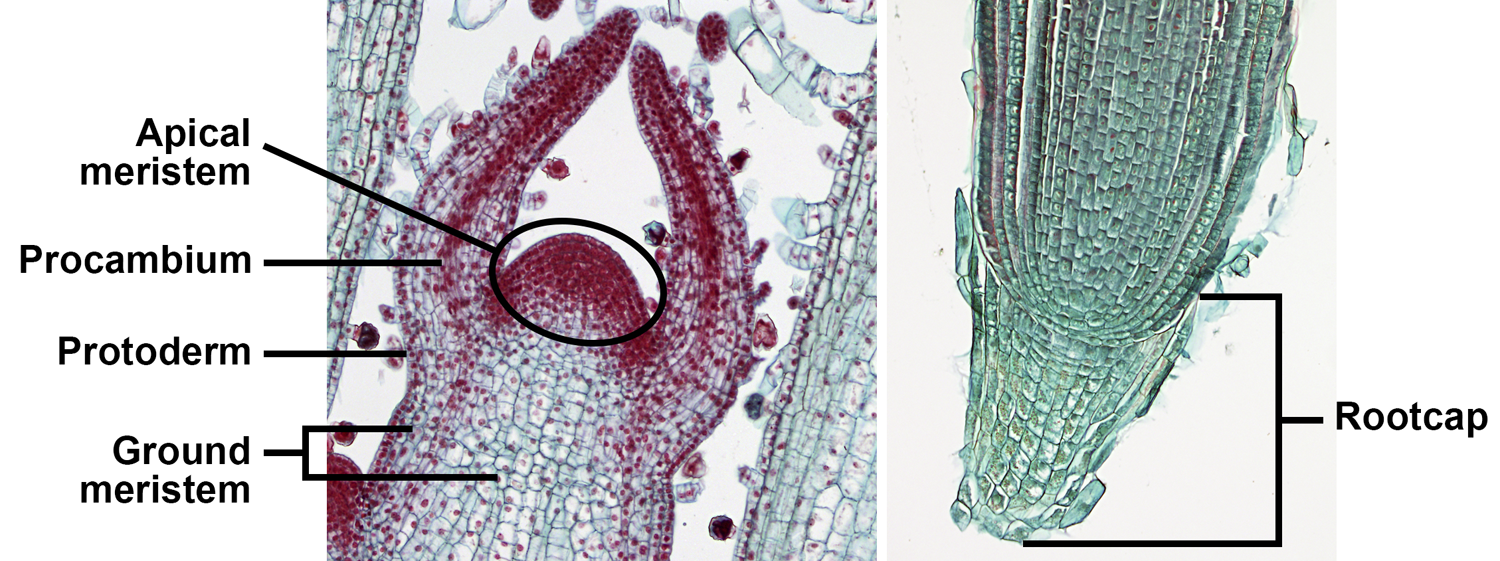
Apical and primary meristems. Left: Longitudinal section of the shoot apex of coleus (Plectranthus, an angiosperm), showing the shoot apical meristem and primary meristems; the horn-like projects to either side of the apical meristem are developing leaves. Right: Longitudinal section of the root apex of wheat (Triticum); the root apical meristem is immediately above the root cap. Credits: Coleus shoot tip and wheat root tip (BlueRidgeKitties, via flickr, CC BY-NC-SA 2.0). Images modified from originals.
LATERAL MERISTEMS (CAMBIA)
Lateral meristems, also called cambia or secondary meristems, are located within roots and stems and increase the diameter of these structures. Growth from cambia is known as secondary growth. Living pteridophytes do not have secondary growth. Seed plants may or may not have secondary growth, depending on the taxon.
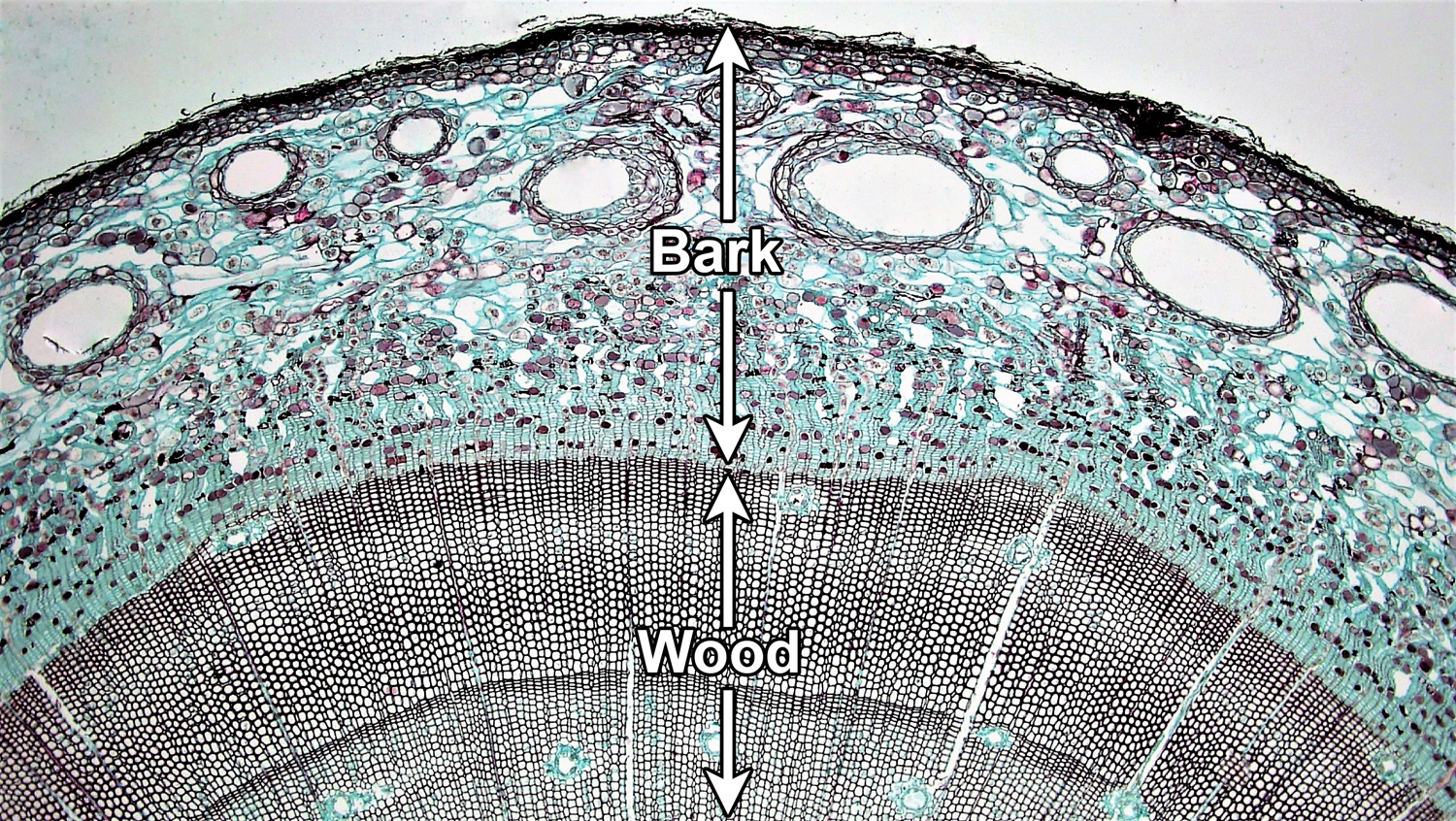
A woody stem or root has two major concentric regions; wood is surrounded by bark. In a typical woody seed plant, two types of lateral meristems contribute cells to these regions. The vascular cambium is a layer of dividing cells between the wood and the bark. It produces vascular tissue to its interior (xylem) and to its exterior (phloem), thus contributing to the growth of both the wood and the bark. The cork cambium develops within the bark and most notably adds layers of protective cork to the bark.
Bark encompasses the entire region outside of the vascular cambium. In addition to tissues produced by the cambia, it may also include tissues produced by primary growth. Wood is made up entirely of xylem (more specifically, secondary xylem, or xylem produced by secondary growth).
Vascular cambium. Cross section of a woody pine (Pinus) stem. The vascular cambium is a layer of dividing cells located between the wood and the bark. Note that the vascular cambium produces cells both to its inside and its outside; the cells are produced in neat rows. In this young stem, much primary tissue is still present in the bark and there is no well-developed cork layer. For a good example of cork, see the cross section of birthwort (Aristolochia) periderm below. Credit: Four year Pinus (Fayette A. Reynolds M.S./Berkshire Community College Bioscience Image Library, via flickr, CC0 1.0/Public Domain Dedication). Image modified from original.
Tissues & tissue systems
Tissues are regions of cells in the plant body that are functionally and physically connected to one another. There are two basic categories of tissues in the plant body, simple and complex. Simple tissues are made up of one type of cell, whereas complex tissues include more than one type of cell. Tissues can also be classified as primary tissues or secondary tissues. Primary tissues are produced by growth from the apical and primary meristems, whereas secondary tissues are produced by growth from the cambia.
Tissues can further be grouped into tissue systems. Tissue systems may include more than one type of tissue. The three tissue systems and their tissues are:
DERMAL TISSUE SYSTEM
The dermal tissue system covers the outer surface of the plant body. The primary dermal tissue is the epidermis, which matures from the protoderm. The epidermis is typically one cell layer thick and primarily made up of epidermal cells. The secondary dermal tissue is the periderm, which is produced by the cork cambium and makes up part of the bark in woody plants. Periderm consists of multiple layers of cells, including outer layers of protective cork cells (which are dead when mature), the cork cambium, and a typically thin inner zone of living cells. The dermal tissues are complex (made up of more than one cell type).
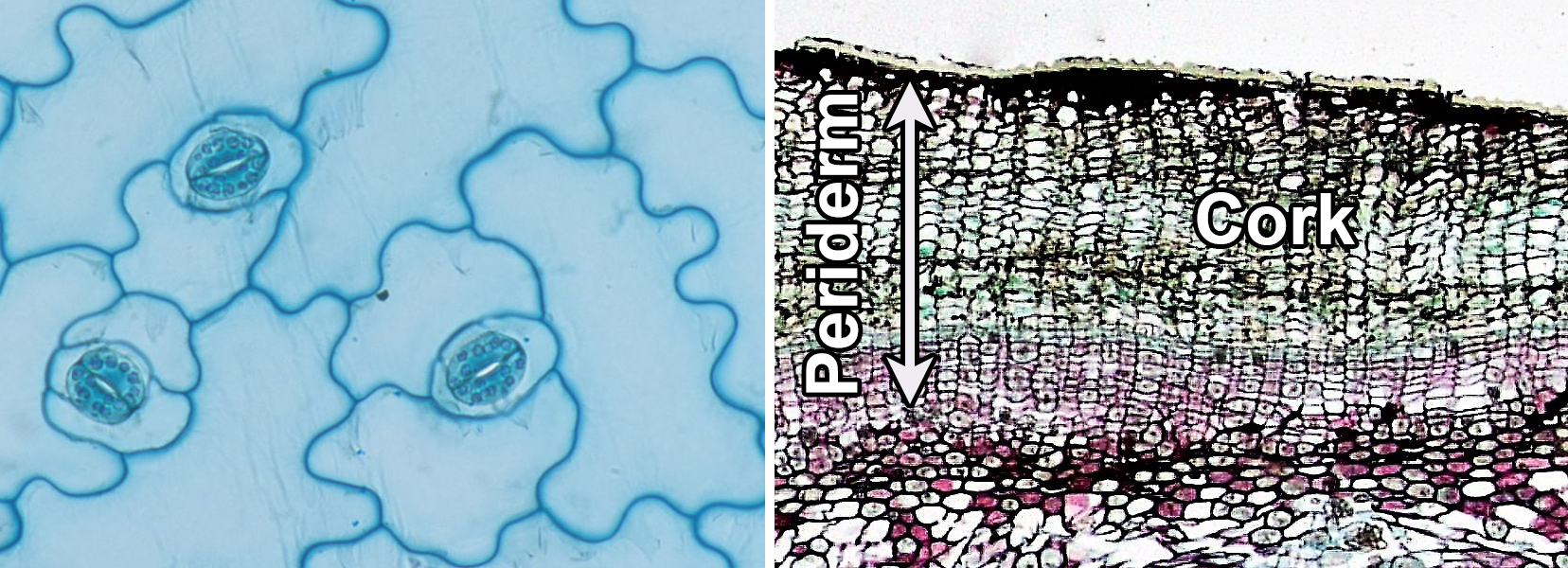
Dermal tissue. Left: Epidermis of kalanchoe (Kalanchoe, an angiosperm) in surface view, showing puzzle-shaped epidermal cells and stomata (pores) surrounded by kidney-shaped guard cells. Right: Periderm of birthwort (Artistolochia, an angiosperm) in cross section, showing thick outer cork. Credits: Surface view of lower epidermis of Kalanchoe leaf (John Adds/Science and Plants for Schools, via flickr, CC BY-NC-SA 2.0); Cross section of Aristolochia stem (Fayette A. Reynolds M.S./Berkshire Community College Bioscience Image Library, via flickr, CC0 1.0/Public Domain Dedication). Images modified from originals.
VASCULAR TISSUE SYSTEM
The vascular tissue system (less often called the fascicular tissue system) conducts food and water through the plant body. The two types of vascular tissue are phloem, or the food-conducting tissue, and xylem, or the water-conducting tissue. Phloem and xylem are complex tissues and include conducting cells and other cell types. The conducting cells are sieve elements in the phloem and tracheary elements in the xylem (a tracheid is a type of tracheary element). Vascular tissue can be primary or secondary, depending on its origin. If it matures from the procambium, it is primary; if it develops from the vascular cambium, it is secondary.
In leaves, vascular tissue is often arranged into one or more veins or vascular bundles (strands) that may form complex networks. The veins are surrounded by a region of ground tissue (discussed below) called the mesophyll.
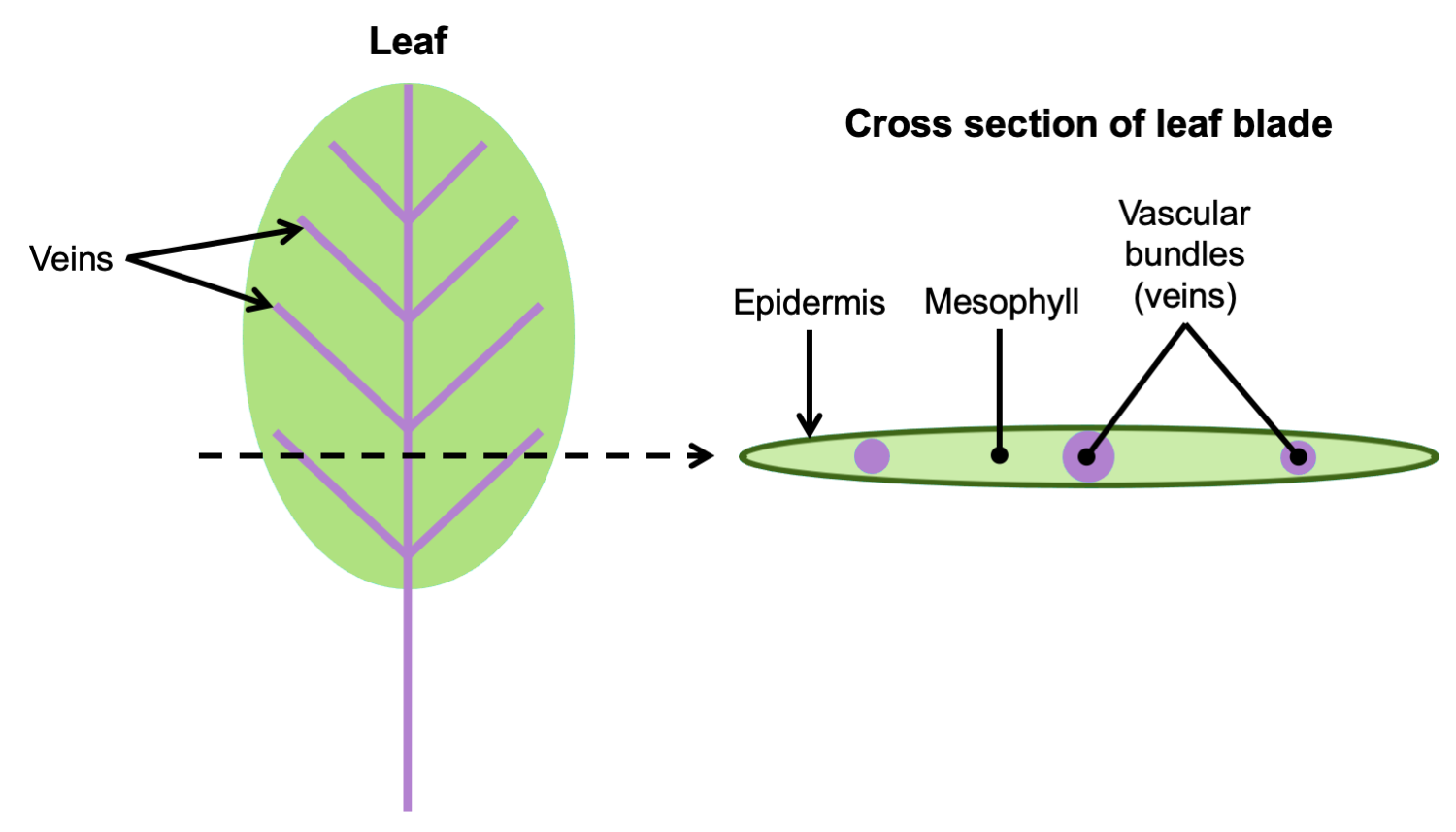
Arrangement of vascular tissue in a leaf. Left: Diagram of a leaf, with the petiole (stalk) and veins in purple; the entire expanded portion of the leaf in which the veins are embedded is the blade. The dotted line indicates where the leaf would be cut to make a cross-section. Right: Cross section of leaf blade showing the vascular bundles embedded in the mesophyll. Credit: E.J. Hermsen (DEAL).
In the stem, the arrangement of primary vascular tissue varies. It can be arranged, for example, in a solid cylinder (protostele), a hollow cylinder surrounding a central pith (siphonostele), or in separate vascular bundles around a pith (eustele). The primary vascular tissue of the root typically takes the form of a protostele (solid cylinder) with a central strand of lobed xylem and phloem between the lobes of xylem.
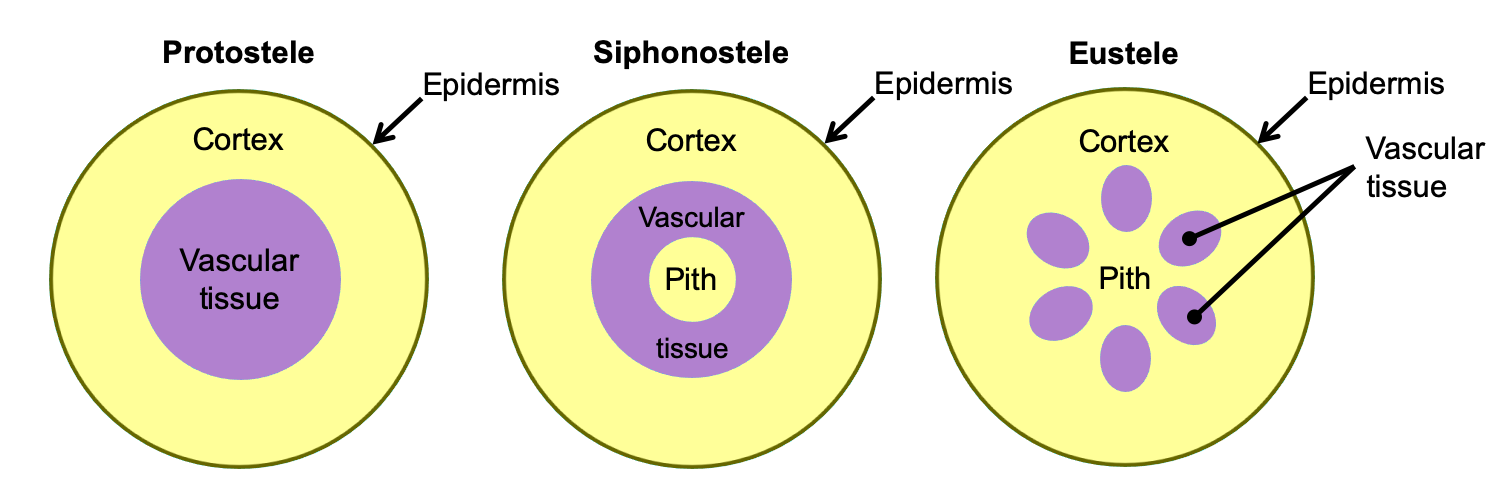
Arrangement of primary vascular tissue in axes (stems and roots). The diagrams above show axes (roots or stems) in cross section (transverse section). Left: Protostele, solid vascular tissue with no pith; this arrangement is typical of roots and of lycophyte stems. Center: Siphonostele, vascular tissue in a ring surrounding a pith; this arrangement is typical of fern stems. Right: Eustele, vascular tissue in bundles surrounding a pith; this arrangement is typical of seed plant stems. Credit: E.J. Hermsen (DEAL).
In stems and roots with secondary growth, the secondary xylem typically forms a continuous ring internal to a continuous ring of secondary phloem. The xylem and phloem are separated by a thin vascular cambium, or layer of undifferentiated dividing cells. Secondary xylem is also known as wood. All tissues external to the vascular cambium are part of the bark. Thus, secondary phloem is part of the bark.
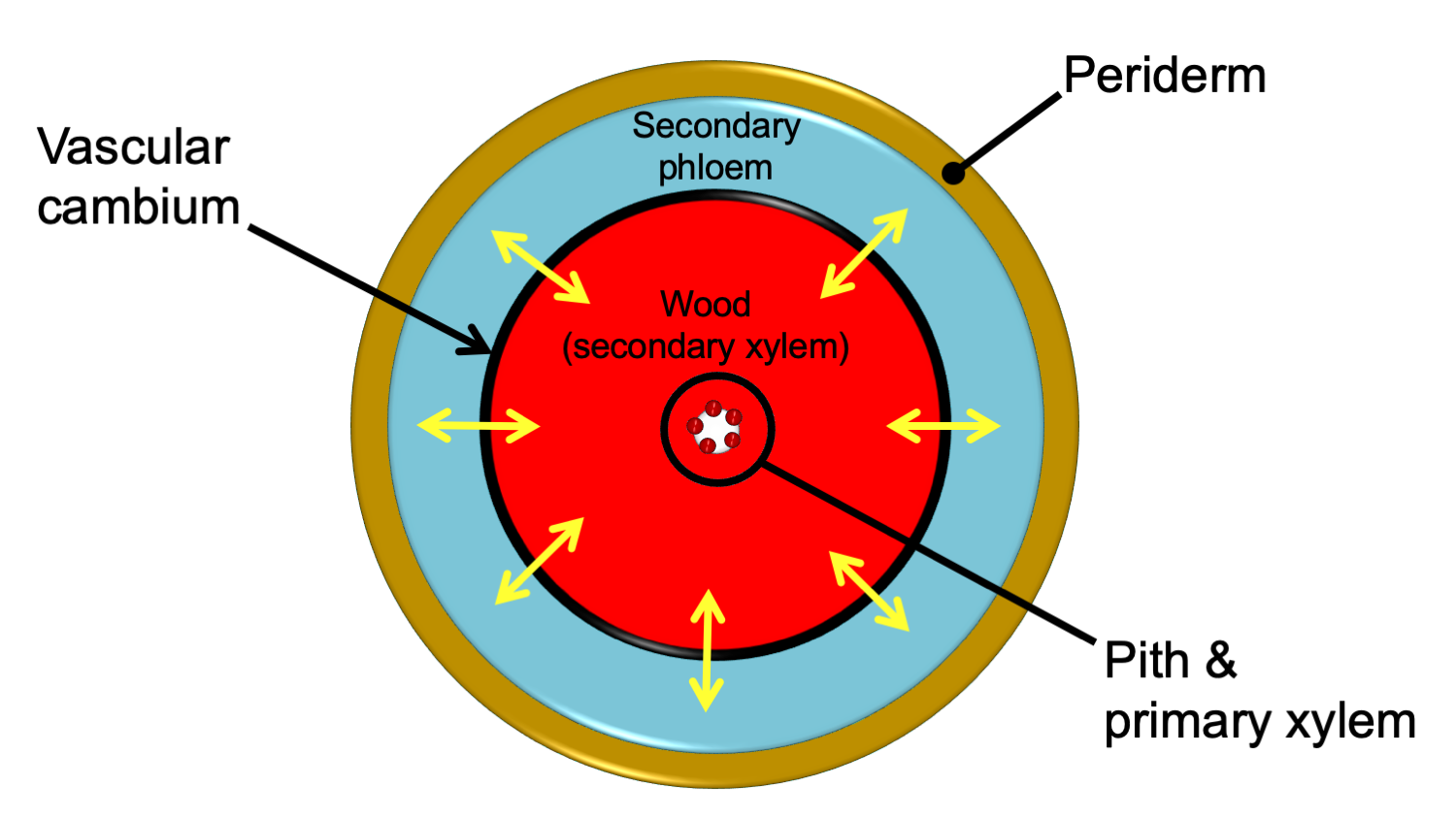
Relative arrangement of secondary tissues in a mature woody stem. This diagram shows the relative arrangement of secondary tissues in a mature seed plant stem in cross section. Note that some of the primary tissues are retained in the center of the woody stem. The yellow arrows show the directions of cell division that add cells to the secondary phloem and secondary xylem. A woody root would be similar in anatomy, except that the center of the stem would be made up of a lobed strand of primary xylem with no pith. In young woody stems, some primary tissues may be retained between the secondary phloem and the periderm (see the cross section of a young pine stem above on this page for an example). These are often crushed and destroyed/sloughed off as the stem ages. Credit: E.J. Hermsen (DEAL).
The ground tissue system (also called the fundamental tissue system) fills in the spaces in the plant body between the dermal and vascular tissues; it develops from the ground meristem. Ground tissue is typically primary tissue. It can be found primarily in the following regions of the plant body:
Cortex: The region between the epidermis and the vascular tissue in the stem and root.
Pith: The region in the center of the stem, internal to the vascular tissue (not always present).
Mesophyll: Occurs between and around the vascular bundles of the leaf.
In addition, ground tissue occurs between the vascular bundles in stems with separate vascular bundles, such as stems with eusteles.
There are three types of ground tissue: parenchyma, collenchyma, and sclerenchyma. They are simple tissues. The cells that make up these tissues share the name of their respective tissue type: parenchyma cells, collenchyma cells, and sclerenchyma cells. Parenchyma cells often have thin cell walls, whereas collenchyma and sclerenchyma cells have thicker cell walls. Collenchyma adds support to non-woody stems and leaves, whereas rigid sclerenchyma cells often function in support or protection. Parenchyma is versatile, and functions in photosynthesis, storage, healing, etc.
Note: Parenchyma cells and sclerenchyma cells do not occur only in the ground tissue system. They can also be found in the vascular tissue.
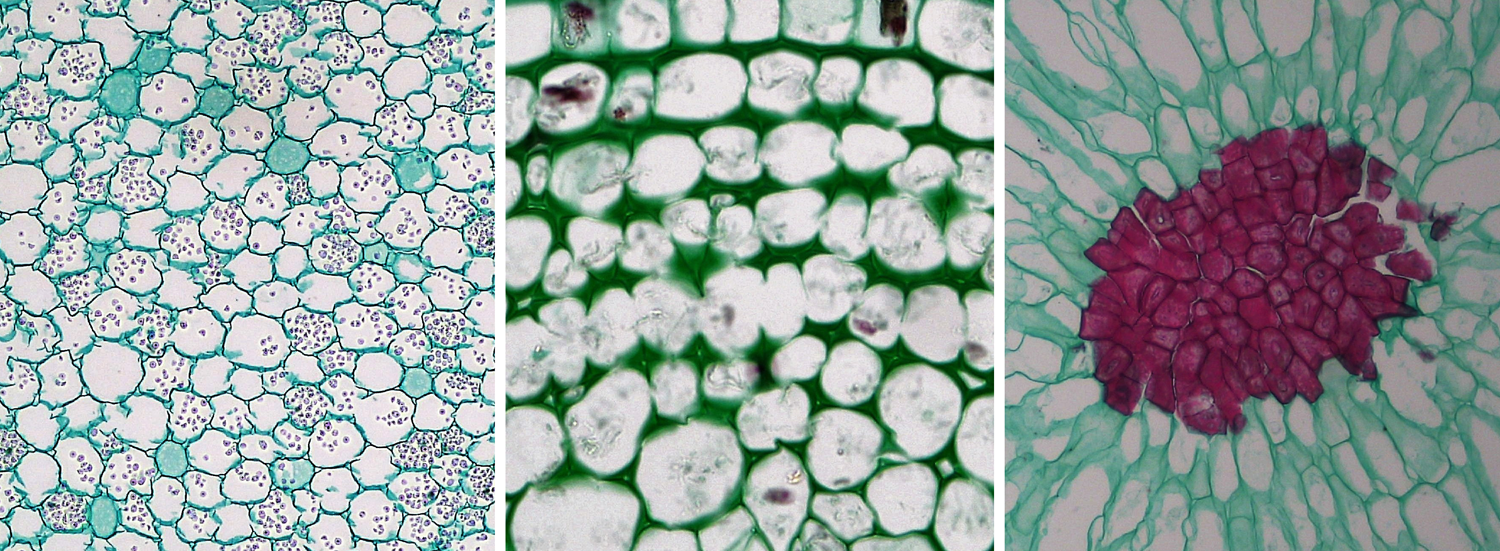
Parenchyma, collenchyma, and sclerenchyma cells. Left: Parenchyma cells from a buttercup (Ranunculus) root, cross section. Center: Collenchyma cells from a sunflower (Helianthus) stem; note the thickened walls of the collenchyma cells, cross section. Right: Sclerenchyma cells from the flesh of a pear (Pyrus); the sclerenchyma cells are a group of stone cells (sclereids) with thick walls that are stained red. Credits: Parenchyma in Ranunculus root, collenchyma in older Helianthus stem, and sclereids in Pyrus (Fayette A. Reynolds M.S./Berkshire Community College Bioscience Image Library, via flickr, CC0 1.0/Public Domain Dedication). Images modified from originals.
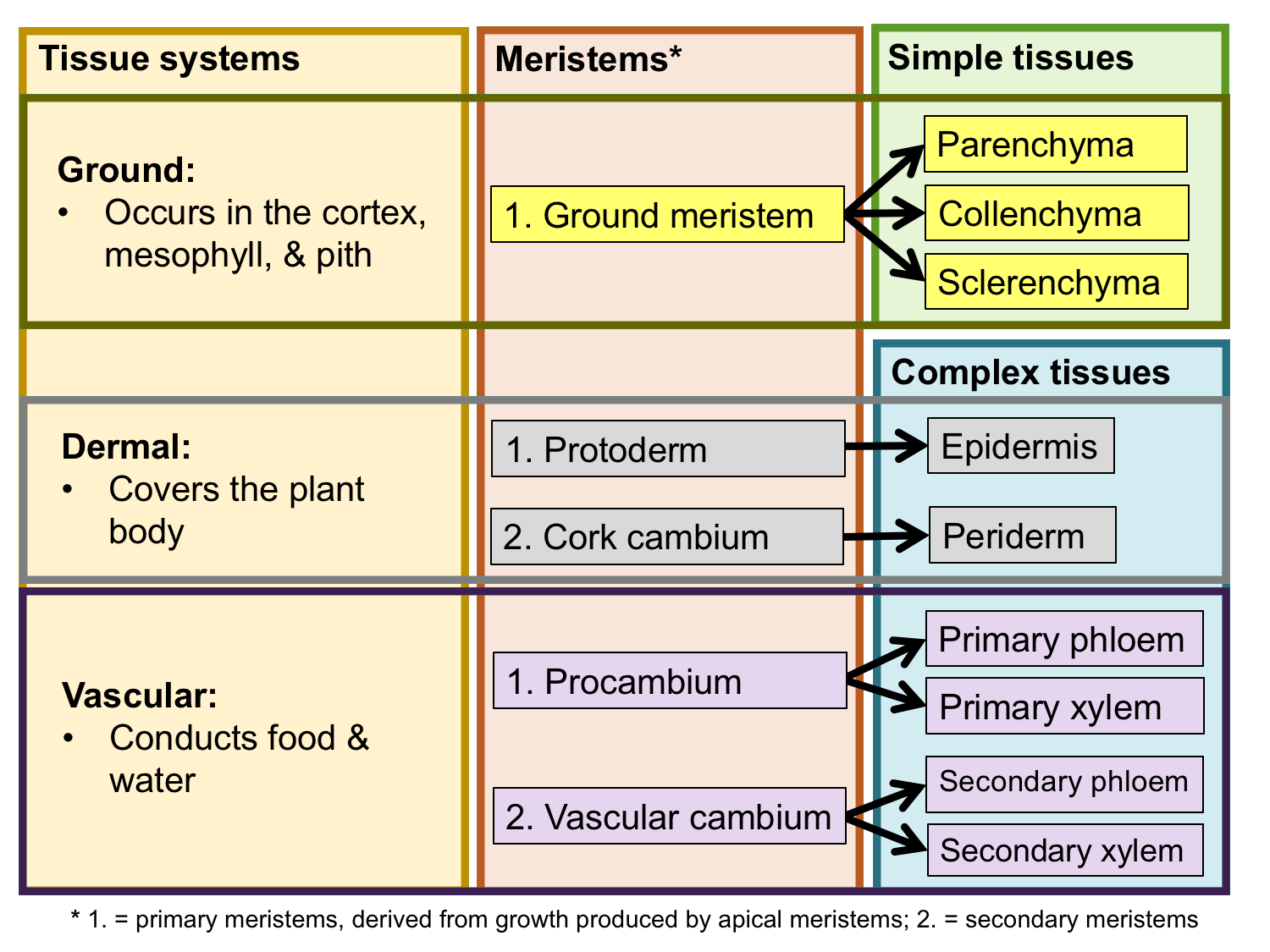
Tissue systems, meristems, and tissues. The chart above summarizes the relationship between the three tissue systems, the meristems that produce them, and the simple and complex tissues that comprise them. Credit: E.J. Hermsen (DEAL).
Selected references & further reading
Note: Free full text is made available by the publisher for items marked with a green star.
Academic articles
* Angiosperm Phylogeny Group, M.W. Chase, M.J.M. Christenhusz, M.F. Fay, J.W. Byng, W.S. Judd, D.E. Soltis, D.J. Mabberley, A.N. Sennikov, P.S. Soltis, and P.F. Stevens. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. https://doi.org/10.1111/boj.12385
* Chaw, S.-M., C.L. Parkinson, Y. Cheng, T.M. Vincent, and J.D. Palmer. 2000. Seed plant phylogeny inferred from all three plant genomes: Monophyly of extant gymnosperms and origin of Gnetales from conifers. PNAS 97: 4086–4091. https://doi.org/10.1073/pnas.97.8.4086
* Davis, C.C., Z. Xi, and S. Mathews. 2014. Plastid phylogenomics and green plant phylogeny: almost full circle but not quite there. BMC Biology 12, article number 11. https://doi.org/10.1186/1741-7007-12-11
* Pteridophyte Phylogeny Group [PPG I]. 2016. A community-derived classification for extant lycophytes and ferns. Journal of Systematics and Evolution 54: 563–603. https://doi.org/10.1111/jse.12229
* Xi, Z., J.S. Rest, and C.C. Davis. 2013. Phylogenomics and coalescent analyses resolve extant seed plant relationships. PLoS ONE 8(11): e80870. https://doi.org/10.1371/journal.pone.0080870
Books & textbooks
Esau, K. 1953. Plant Anatomy. John Wiley & Sons, New York.
Esau, K. 1977. Anatomy of Seed Plants, 2nd. ed. John Wiley & Sons, New York.
Evert, R.F., and S.E. Eichhorn. 2013. Raven Biology of Plants, 8th ed. W.H. Freeman and Co., New York, New York.
Peterson, R.L., C.A. Peterson, and L.H. Melville. 2008. Teaching Plant Anatomy through Creative Laboratory Exercises. NRC Research Press, Ottawa, Ontario, Canada.
Web resources: plant anatomy images
* Berkshire Community College Bioscience Image Library: Plant tissues and organs: http://blogs.berkshirecc.edu/bccoer/plants/
* Botanical Society of America Plant Image Collection: Plant anatomy: https://cms.botany.org/media/collection/id.24.html
* Cornell University Plant Anatomy Collection (CUPAC): http://cupac.bh.cornell.edu/
* Plant Anatomy Laboratory: Micrographs of plant cells and tissues, with explanatory text (J.D. Mauseth): http://www.sbs.utexas.edu/mauseth/weblab/
* Photographic Atlas of Plant Anatomy (J.D. Curtis, N.R. Lersten, and M.D. Nowak, revised by D. Hillier and J. Hardy): https://botweb.uwsp.edu/Anatomy/
* The Swingle Plant Anatomy Reference Collection (University of Miami): http://swingle.miami.edu/plantReference.html
* University of California Santa Barbara: Plant Anatomy (images by Vernon I. Cheadle and Katherine Esau): https://www.ccber.ucsb.edu/collections/botanical/plant_anatomy
* University of Wisconsin-Madison Botany Department Teaching Collection. https://uwdc.library.wisc.edu/collections/genbot/
Content usage
Usage of text and images created for DEAL: Text on this page was written by Elizabeth J. Hermsen. Original written content created by E.J. Hermsen for the Digital Encyclopedia of Ancient Life that appears on this page is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Original images and diagrams created by E.J. Hermsen are also licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Content sourced from other websites: Attribution, source webpage, and licensing information or terms of use are indicated for images sourced from other websites in the figure caption below the relevant image. See original sources for further details. Attribution and source webpage are indicated for embedded videos. See original sources for terms of use. Reproduction of an image or video on this page does not imply endorsement by the author, creator, source website, publisher, and/or copyright holder.
Adapted images. Images that have been adapted or remixed for DEAL (e.g., labelled images, multipanel figures) are governed by the terms of the original image license(s) covering attribution, general reuse, and commercial reuse. DEAL places no further restrictions above or beyond those of the original creator(s) and/or copyright holder(s) on adapted images, although we ask that you credit DEAL if reusing an adapted image from the DEAL website. Please note that some DEAL figures may only be reused with permission of the creator(s) or copyright holder(s) of the original images. Consult the individual image credits for further details.
First released 30 March 2020; last updated 31 March 2020.
Bibliografia
https://elifesciences.org/articles/38827
https://pdfs.semanticscholar.org/afdf/29ad6572ba9e6355a877ad1d06fe72951208.pdf?_ga=2.245951116.946476401.1589329699-284509423.1587934422
https://www.michaelwachtler.com/download/Origin_angiosperms.pdf



0 comentários:
Postar um comentário